Yeungnam Univ J Med.
2019 Sep;36(3):163-182. 10.12701/yujm.2019.00227.
Intraoperative consultation for ovarian tumors
- Affiliations
-
- 1Department of Pathology, Jinju Korea Hospital, Jinju, Korea. iskim@korea.ac.kr
- KMID: 2460185
- DOI: http://doi.org/10.12701/yujm.2019.00227
Abstract
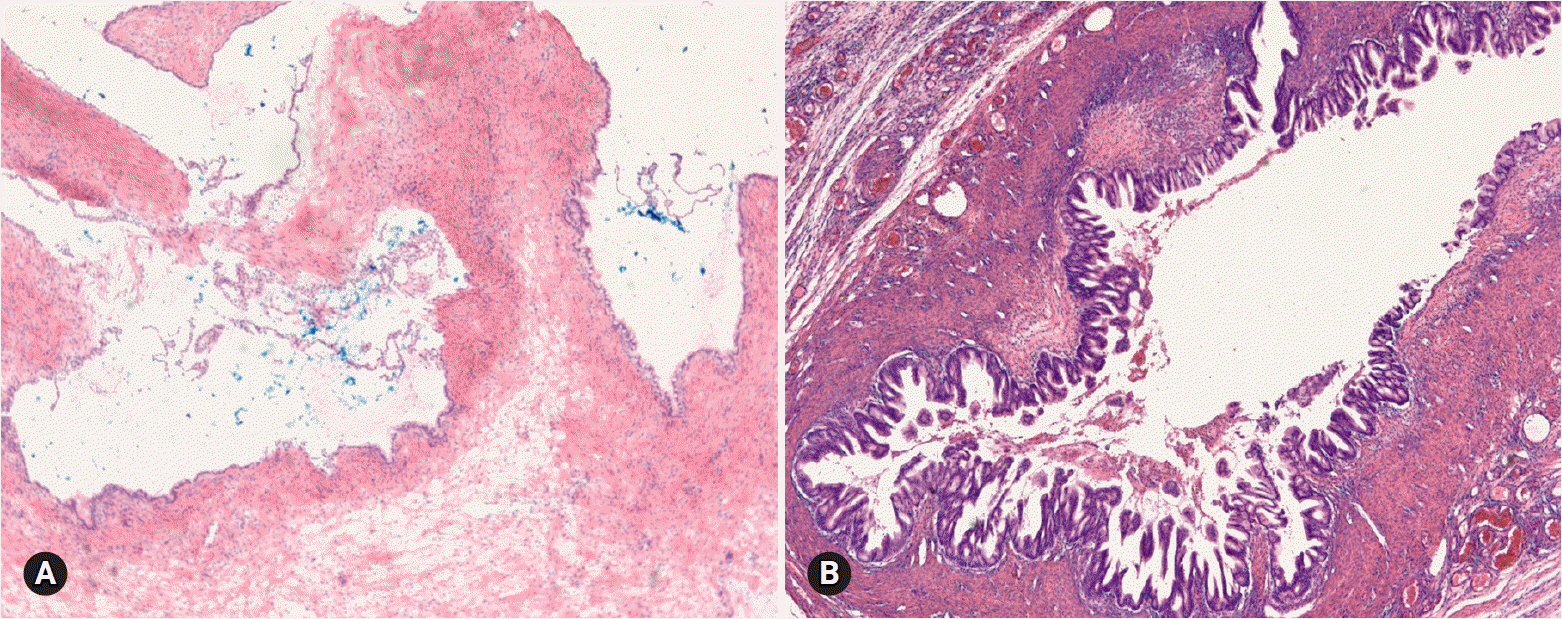
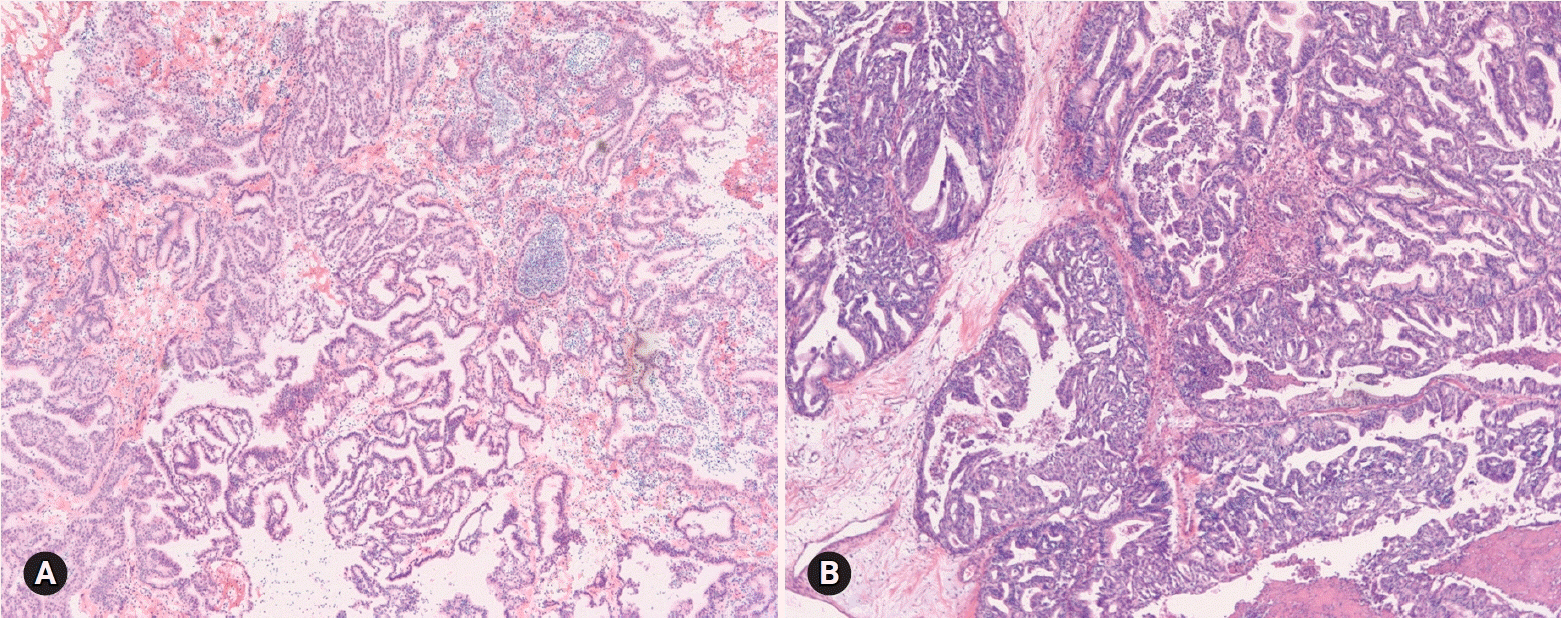
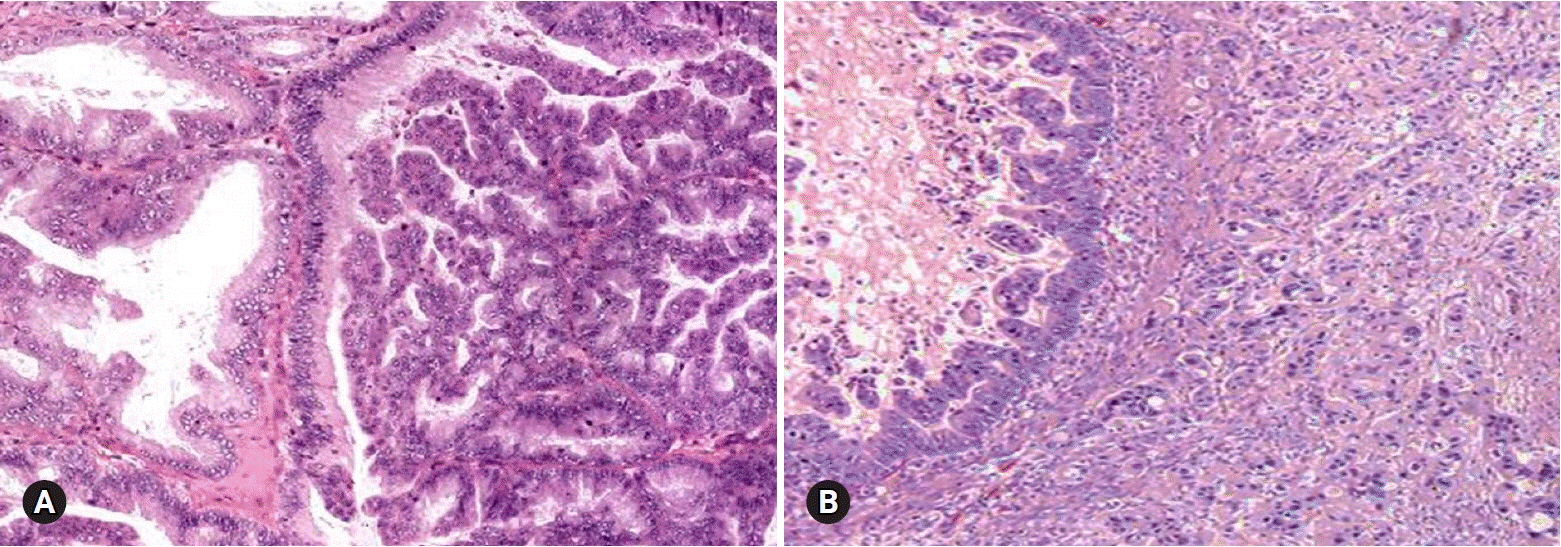
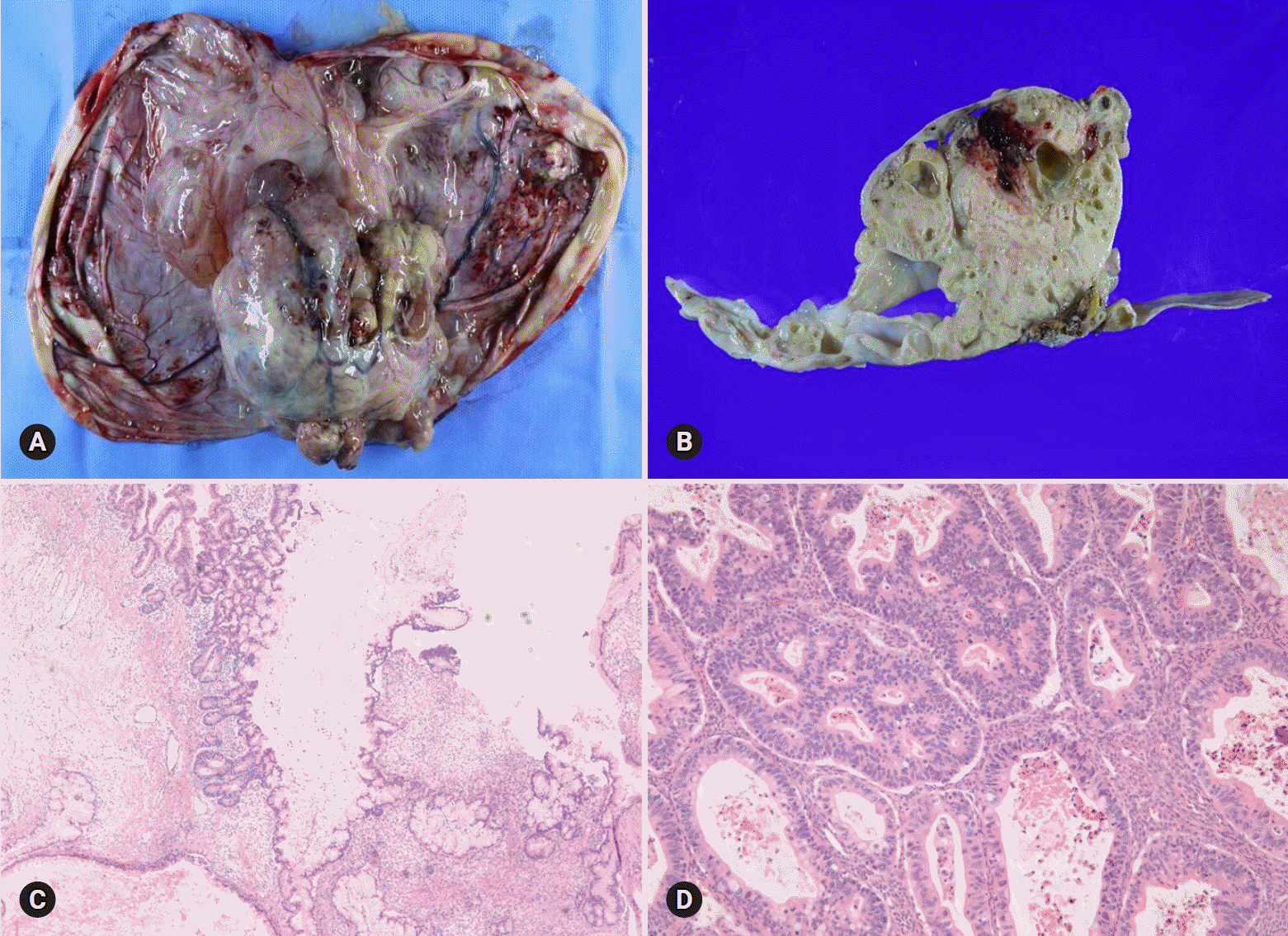
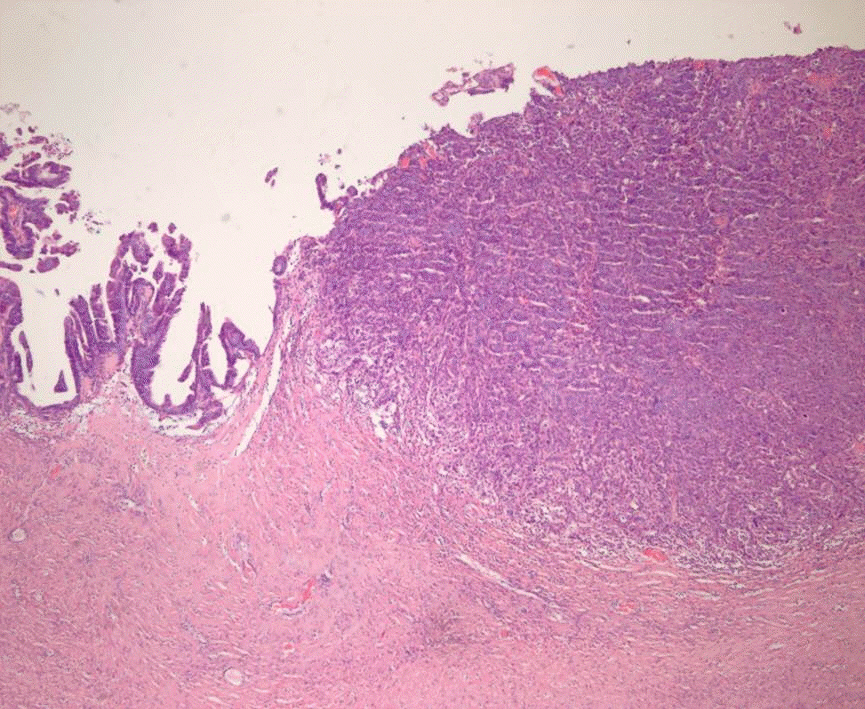
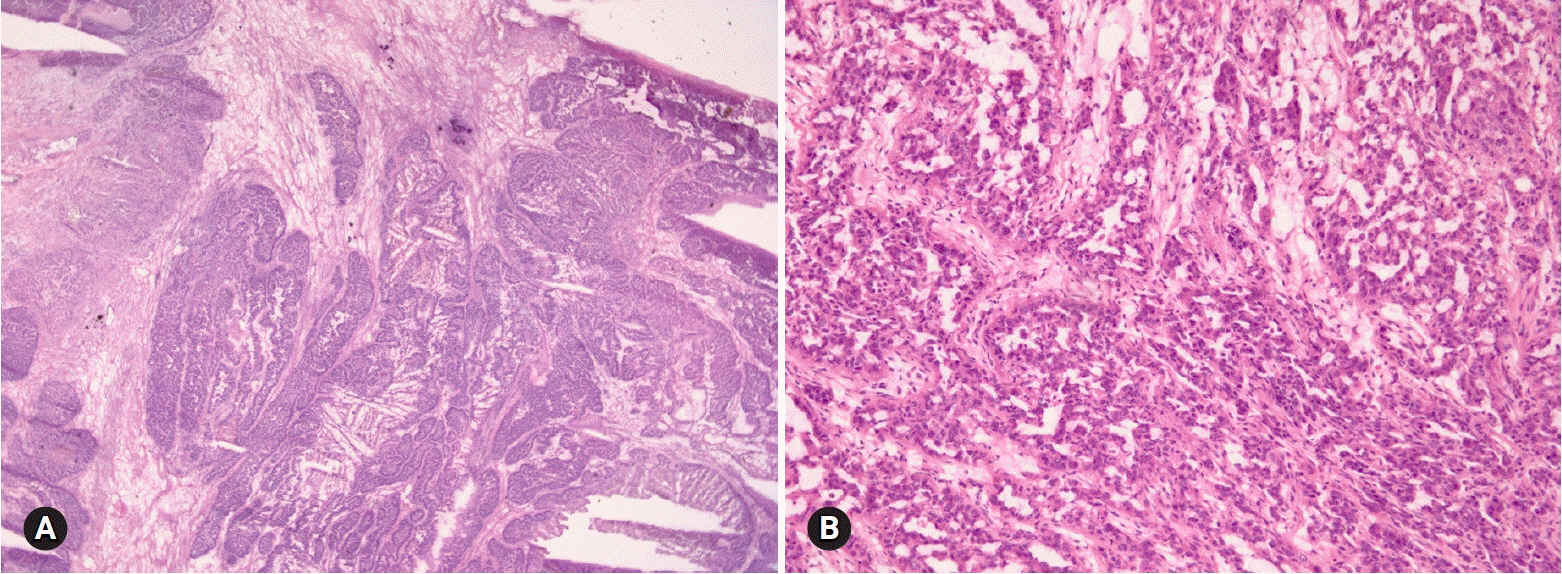
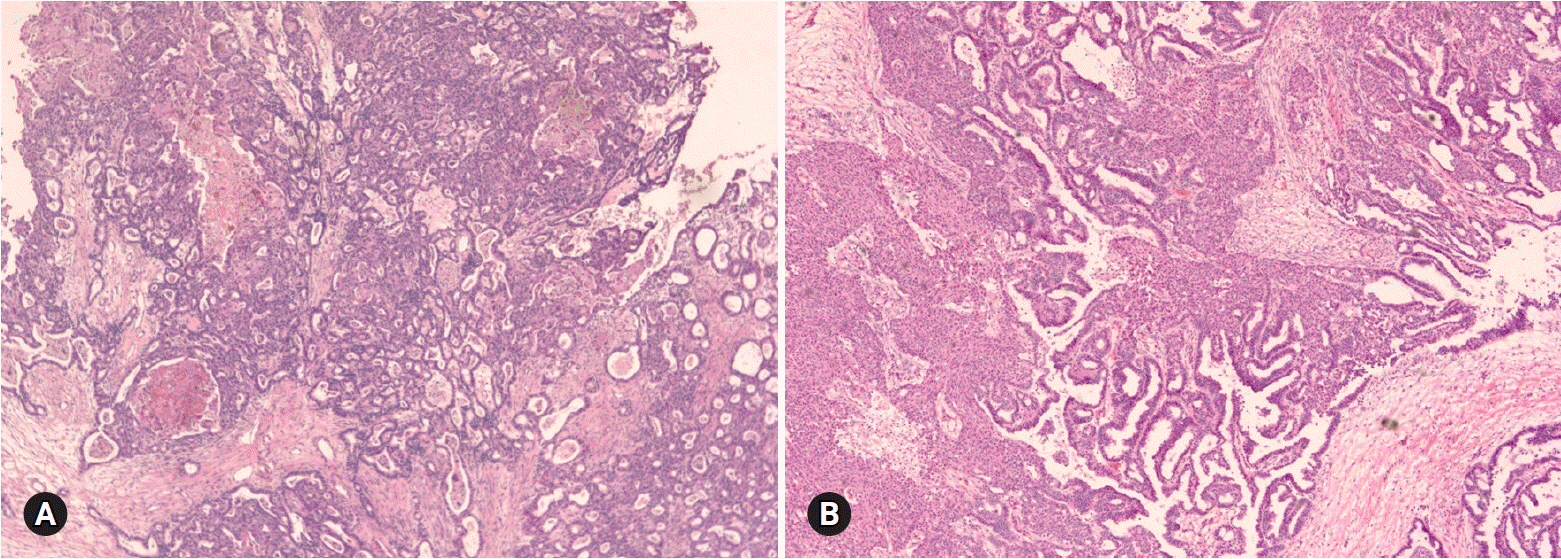
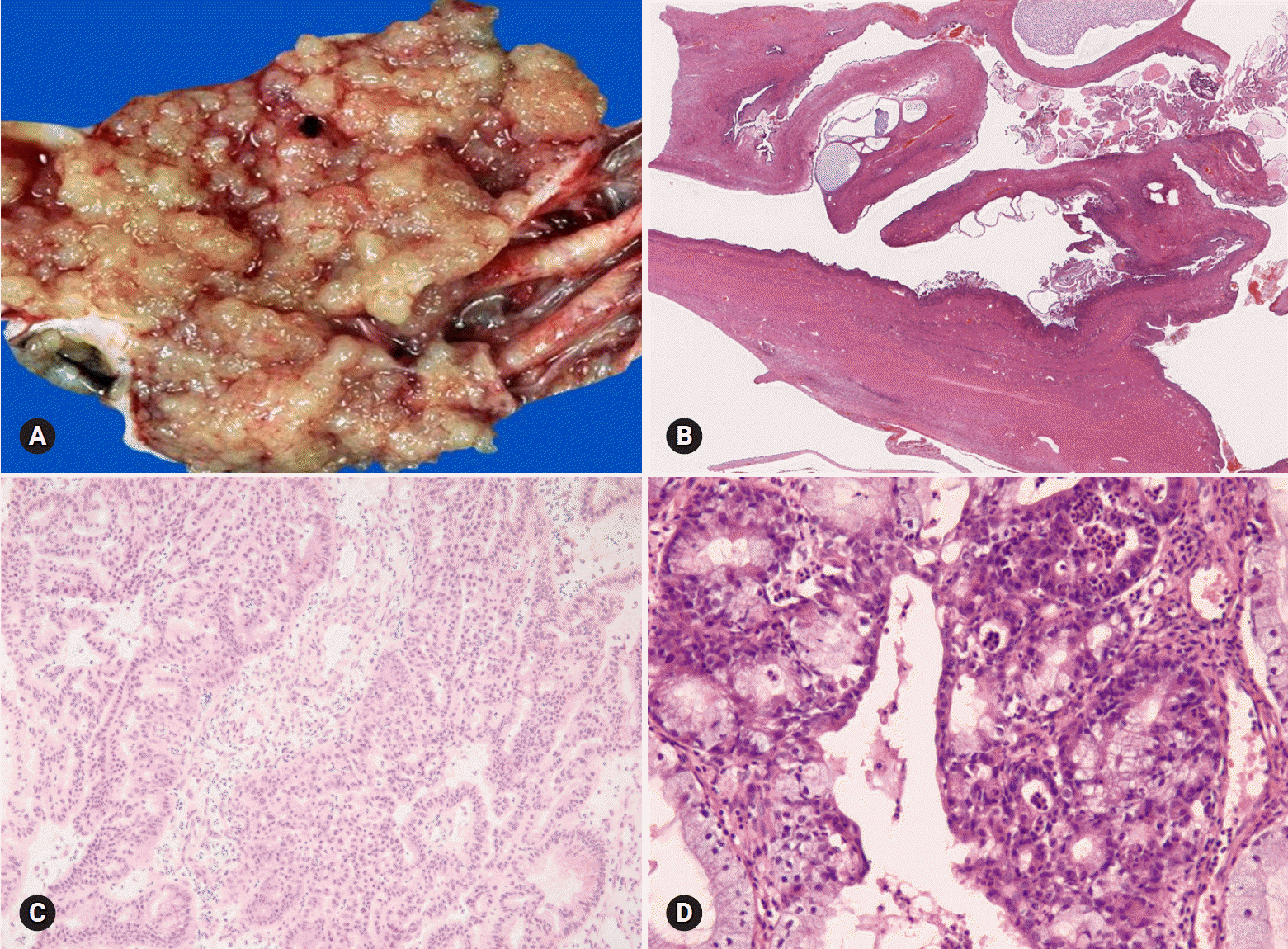
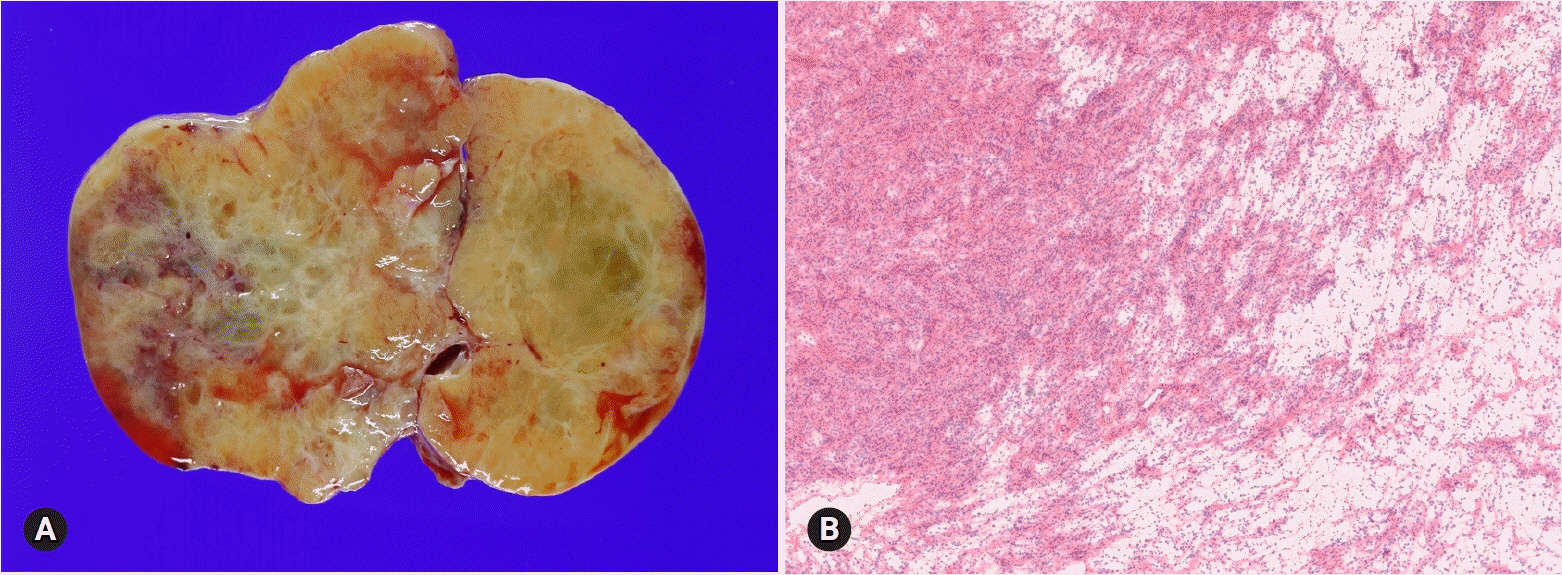
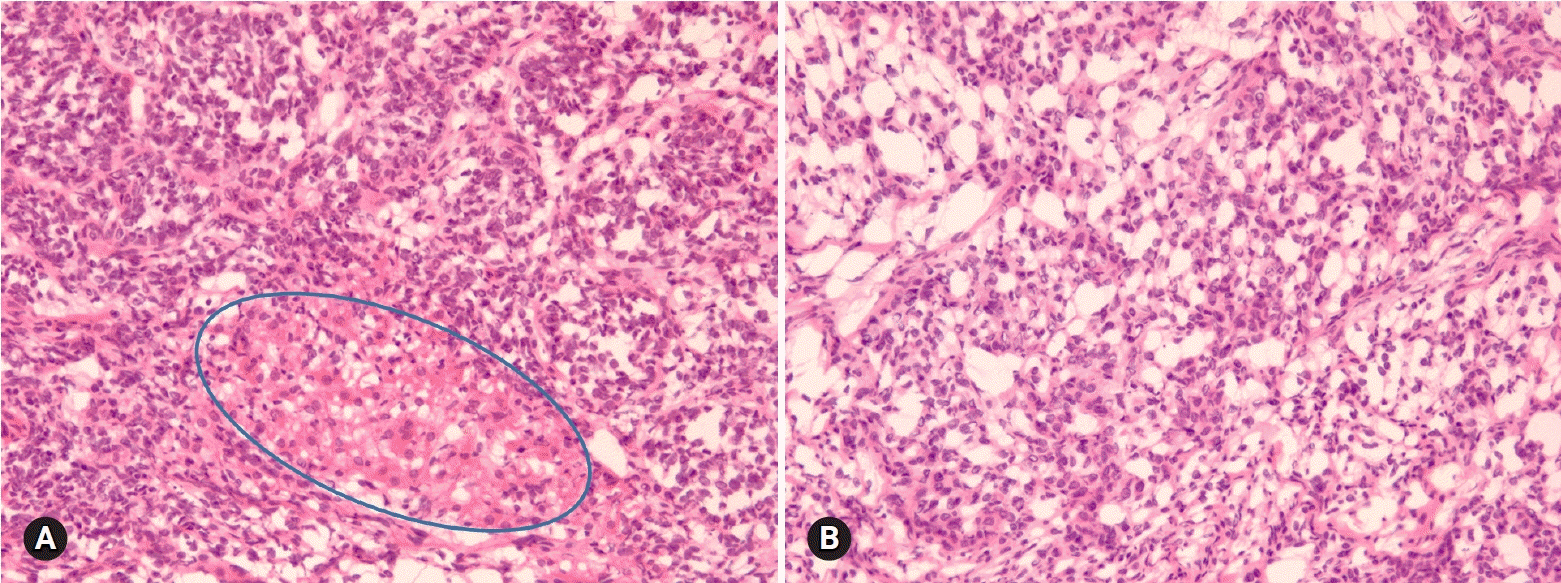
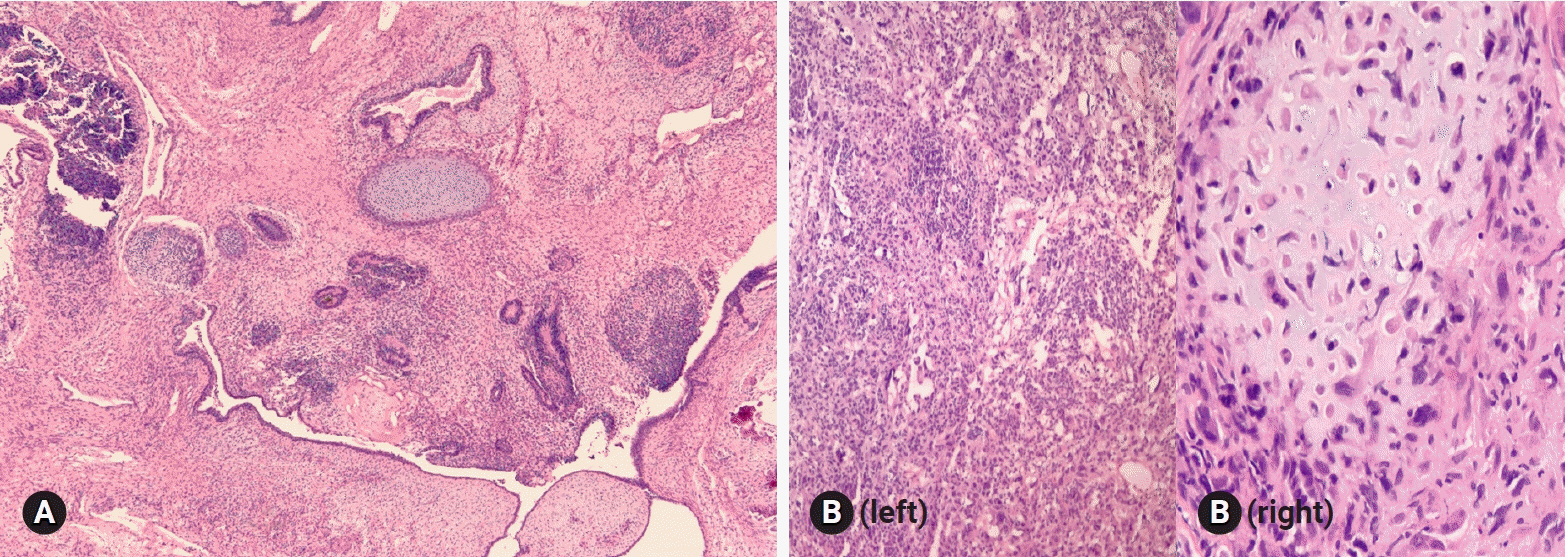
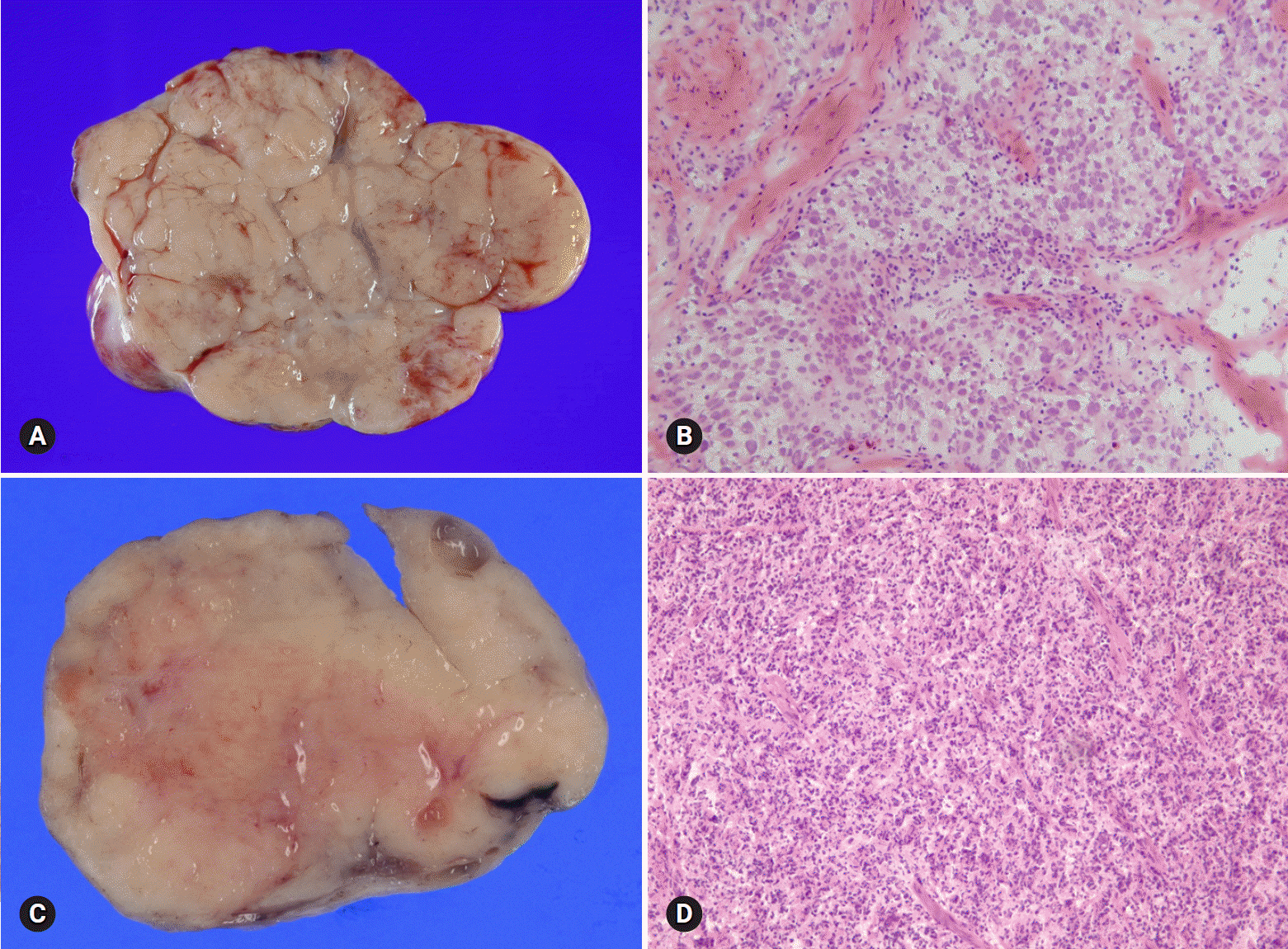
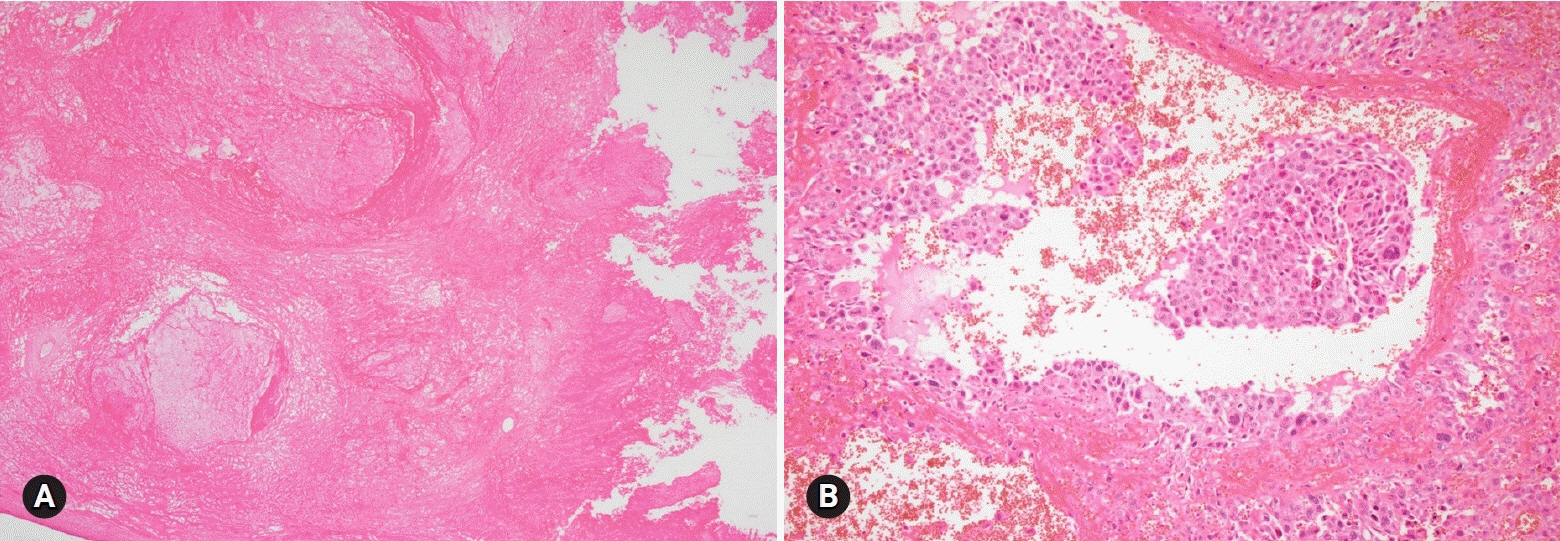
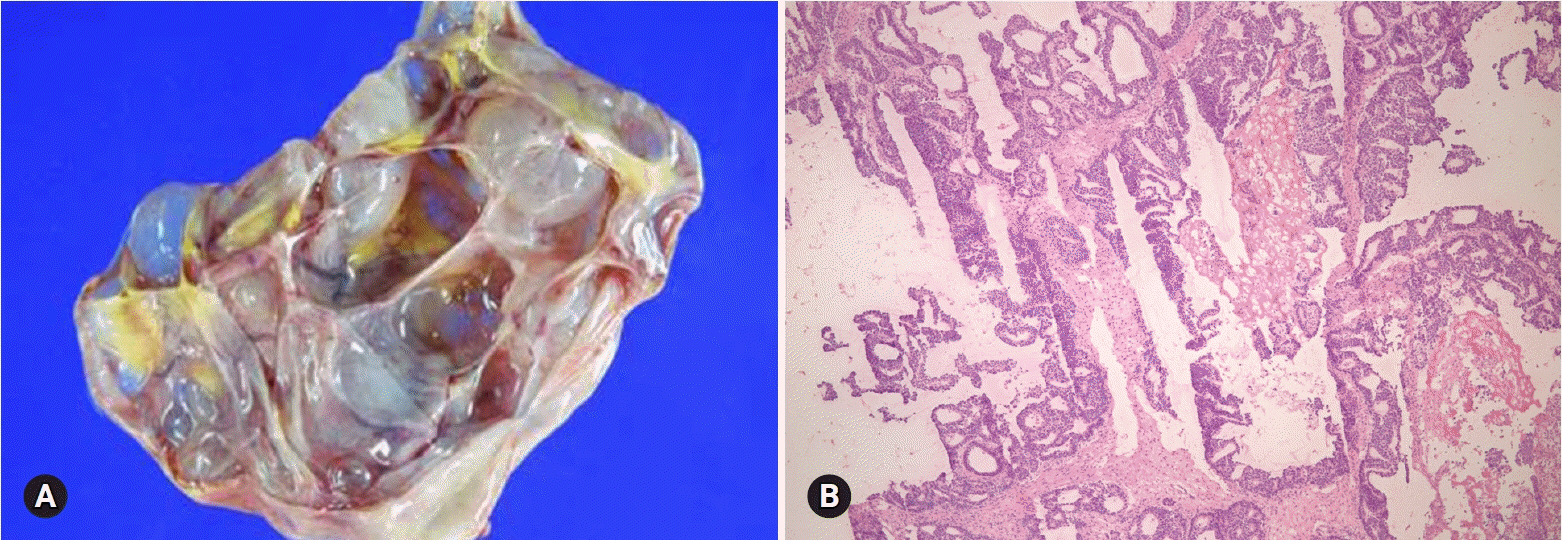
- The primary function of intraoperative frozen consultation is to provide an as accurate and prompt diagnosis as possible during surgery and to guide the surgeon in further management. However, the evaluation of frozen section (FS) is sometimes difficult because of suboptimal tissue quality and frozen artifacts compared with routinely processed tissue section. The pathologist responsible for the FS diagnosis requires experience and good judgment. Ovarian tumors are a heterogeneous group of tumors including primary surface epithelial tumors, germ cell tumors and sex cord-stromal tumors, secondary tumors, and other groups of tumors of uncertain histogenesis or nonspecific stroma. Intraoperative FS is a very important and reliable tool that guides the surgical management of ovarian tumors. In this review, the diagnostic key points for the pathologist and the implication of the FS diagnosis on the operator's decisions are discussed.
MeSH Terms
Figure
Reference
-
References
1. Gal AA, Cagle PT. The 100-year anniversary of the description of the frozen section procedure. JAMA. 2005; 294:3135–7.
Article2. Krishnamurthy S, Meric-Bernstam F, Lucci A, Hwang RF, Kuerer HM, Babiera G, et al. A prospective study comparing touch imprint cytology, frozen section analysis, and rapid cytokeratin immunostain for intraoperative evaluation of axillary sentinel lymph nodes in breast cancer. Cancer. 2009; 115:1555–62.
Article3. Novis DA, Zarbo RJ. Interinstitutional comparison of frozen section turnaround time. A College of American Pathologists Q-Probes study of 32868 frozen sections in 700 hospitals. Arch Pathol Lab Med. 1997; 121:559–67.4. Kurman RJ; International Agency for Research on Cancer (IARC). WHO classification of tumours of female reproductive organs. 4th ed. Lyon: International Agency for Research on Cancer;2014.5. Bige O, Demir A, Saygili U, Gode F, Uslu T, Koyuncuoglu M. Frozen section diagnoses of 578 ovarian tumors made by pathologists with and without expertise on gynecologic pathology. Gynecol Oncol. 2011; 123:43–6.
Article6. Malipatil R, Crasta JA. How accurate is intraoperative frozen section in the diagnosis of ovarian tumors? J Obstet Gynaecol Res. 2013; 39:710–3.
Article7. Rodríguez IM, Prat J. Mucinous tumors of the ovary: a clinicopathologic analysis of 75 borderline tumors (of intestinal type) and carcinomas. Am J Surg Pathol. 2002; 26:139–52.8. Lee KR, Young RH. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases. Am J Surg Pathol. 2003; 27:281–92.9. Bagué S, Rodríguez IM, Prat J. Sarcoma-like mural nodules in mucinous cystic tumors of the ovary revisited: a clinicopathologic analysis of 10 additional cases. Am J Surg Pathol. 2002; 26:1467–76.10. Provenza C, Young RH, Prat J. Anaplastic carcinoma in mucinous ovarian tumors: a clinicopathologic study of 34 cases emphasizing the crucial impact of stage on prognosis, their histologic spectrum, and overlap with sarcomalike mural nodules. Am J Surg Pathol. 2008; 32:383–9.11. Seidman JD, Soslow RA, Vang R, Berman JJ, Stoler MH, Sherman ME, et al. Borderline ovarian tumors: diverse contemporary viewpoints on terminology and diagnostic criteria with illustrative images. Hum Pathol. 2004; 35:918–33.12. McKenney JK, Balzer BL, Longacre TA. Patterns of stromal invasion in ovarian serous tumors of low malignant potential (borderline tumors): a reevaluation of the concept of stromal microinvasion. Am J Surg Pathol. 2006; 30:1209–21.
Article13. Bell DA, Weinstock MA, Scully RE. Peritoneal implants of ovarian serous borderline tumors. Histologic features and prognosis. Cancer. 1988; 62:2212–22.
Article14. Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004; 28:496–504.
Article15. Roth LM, Emerson RE, Ulbright TM. Ovarian endometrioid tumors of low malignant potential: a clinicopathologic study of 30 cases with comparison to well-differentiated endometrioid adenocarcinoma. Am J Surg Pathol. 2003; 27:1253–9.16. Bell KA, Kurman RJ. A clinicopathologic analysis of atypical proliferative (borderline) tumors and well-differentiated endometrioid adenocarcinomas of the ovary. Am J Surg Pathol. 2000; 24:1465–79.
Article17. Brescia RJ, Dubin N, Demopoulos RI. Endometrioid and clear cell carcinoma of the ovary. Factors affecting survival. Int J Gynecol Pathol. 1989; 8:132–8.18. Zaino RJ, Unger ER, Whitney C. Synchronous carcinomas of the uterine corpus and ovary. Gynecol Oncol. 1984; 19:329–35.
Article19. Roth LM, Langley FA, Fox H, Wheeler JE, Czernobilsky B. Ovarian clear cell adenofibromatous tumors. Benign, of low malignant potential, and associated with invasive clear cell carcinoma. Cancer. 1984; 53:1156–63.
Article20. Scarfone G, Bergamini A, Noli S, Villa A, Cipriani S, Taccagni G, et al. Characteristics of clear cell ovarian cancer arising from endometriosis: a two center cohort study. Gynecol Oncol. 2014; 133:480–4.
Article21. Irving JA, Alkushi A, Young RH, Clement PB. Cellular fibromas of the ovary: a study of 75 cases including 40 mitotically active tumors emphasizing their distinction from fibrosarcoma. Am J Surg Pathol. 2006; 30:929–38.
Article22. Bhat RA, Lim YK, Chia YN, Yam KL. Sertoli-Leydig cell tumor of the ovary: analysis of a single institution database. J Obstet Gynaecol Res. 2013; 39:305–10.
Article23. van Meurs HS, Bleeker MC, van der Velden J, Overbeek LI, Kenter GG, Buist MR. The incidence of endometrial hyperplasia and cancer in 1031 patients with a granulosa cell tumor of the ovary: long-term follow-up in a population-based cohort study. Int J Gynecol Cancer. 2013; 23:1417–22.24. Young RH, Dickersin GR, Scully RE. Juvenile granulosa cell tumor of the ovary. A clinicopathological analysis of 125 cases. Am J Surg Pathol. 1984; 8:575–96.25. Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. 2003; 21:1180–9.
Article26. Iavazzo C, Gkegkes ID, Vrachnis N. Fertility sparing management and pregnancy in patients with granulosa cell tumour of the ovaries. J Obstet Gynaecol. 2015; 35:331–5.
Article27. Young RH, Scully RE. Ovarian Sertoli-Leydig cell tumors. A clinicopathological analysis of 207 cases. Am J Surg Pathol. 1985; 9:543–69.28. Gui T, Cao D, Shen K, Yang J, Zhang Y, Yu Q, et al. A clinicopathological analysis of 40 cases of ovarian Sertoli-Leydig cell tumors. Gynecol Oncol. 2012; 127:384–9.
Article29. Norris HJ, Zirkin HJ, Benson WL. Immature (malignant) teratoma of the ovary: a clinical and pathologic study of 58 cases. Cancer. 1976; 37:2359–72.
Article30. Stamp GW, McConnell EM. Malignancy arising in cystic ovarian teratomas. A report of 24 cases. Br J Obstet Gynaecol. 1983; 90:671–5.31. Eskander RN, Randall LM, Berman ML, Tewari KS, Disaia PJ, Bristow RE. Fertility preserving options in patients with gynecologic malignancies. Am J Obstet Gynecol. 2011; 205:103–10.
Article32. Nogales FF, Preda O, Nicolae A. Yolk sac tumours revisited. A review of their many faces and names. Histopathology. 2012; 60:1023–33.
Article33. Roth LM, Talerman A, Levy T, Sukmanov O, Czernobilsky B. Ovarian yolk sac tumors in older women arising from epithelial ovarian tumors or with no detectable epithelial component. Int J Gynecol Pathol. 2011; 30:442–51.
Article34. Oliva E, Andrada E, Pezzica E, Prat J. Ovarian carcinomas with choriocarcinomatous differentiation. Cancer. 1993; 72:2441–6.
Article35. Ulbright TM, Roth LM, Stehman FB. Secondary ovarian neoplasia. A clinicopathologic study of 35 cases. Cancer. 1984; 53:1164–74.
Article36. Mazur MT, Hsueh S, Gersell DJ. Metastases to the female genital tract. Analysis of 325 cases. Cancer. 1984; 53:1978–84.
Article37. Yemelyanova AV, Vang R, Judson K, Wu LS, Ronnett BM. Distinction of primary and metastatic mucinous tumors involving the ovary: analysis of size and laterality data by primary site with reevaluation of an algorithm for tumor classification. Am J Surg Pathol. 2008; 32:128–38.
Article38. Young RH. From krukenberg to today: the ever present problems posed by metastatic tumors in the ovary: part I. Historical perspective, general principles, mucinous tumors including the krukenberg tumor. Adv Anat Pathol. 2006; 13:205–27.39. Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol. 2006; 30:277–99.40. Young RH, Gilks CB, Scully RE. Mucinous tumors of the appendix associated with mucinous tumors of the ovary and pseudomyxoma peritonei. A clinicopathological analysis of 22 cases supporting an origin in the appendix. Am J Surg Pathol. 1991; 15:415–29.41. Stewart CJ, Ardakani NM, Doherty DA, Young RH. An evaluation of the morphologic features of low-grade mucinous neoplasms of the appendix metastatic in the ovary, and comparison with primary ovarian mucinous tumors. Int J Gynecol Pathol. 2014; 33:1–10.
Article42. Ronnett BM, Yemelyanova AV, Vang R, Gilks CB, Miller D, Gravitt PE, et al. Endocervical adenocarcinomas with ovarian metastases: analysis of 29 cases with emphasis on minimally invasive cervical tumors and the ability of the metastases to simulate primary ovarian neoplasms. Am J Surg Pathol. 2008; 32:1835–53.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of E-cadherin in Benign, Borderline, and Malignant Ovarian Epithelial Tumors
- Mucinous Tumors of the Appendix Associated with Mucinous Tumors of the Ovary and Pseudomyxoma Peritonei: A Clinicopathologic Analysis of 5 Cases Supporting an Appendiceal Origin
- Diagnostic Significance of Serum Tumor Markers in Paitents with Ovarian Tumors
- Cyclooxygenase-2 Expression in Ovarian Tumors
- Treatment of the Ovarian Germ Cell Tumors