Investig Magn Reson Imaging.
2019 Sep;23(3):202-209. 10.13104/imri.2019.23.3.202.
Ultrashort Echo Time MRI (UTE-MRI) Quantifications of Cortical Bone Varied Significantly at Body Temperature Compared with Room Temperature
- Affiliations
-
- 1Department of Radiology, University of California, San Diego, United States. jiangdu@ucsd.edu, sjerban@ucsd.edu
- 2Radiology Service, VA San Diego Healthcare System, San Diego, United States.
- KMID: 2459874
- DOI: http://doi.org/10.13104/imri.2019.23.3.202
Abstract
- PURPOSE
To investigate the temperature-based differences of cortical bone ultrashort echo time MRI (UTE-MRI) biomarkers between body and room temperatures. Investigations of ex vivo UTE-MRI techniques were performed mostly at room temperature however, it is noted that the MRI properties of cortical bone may differ in vivo due to the higher temperature which exists as a condition in the live body.
MATERIALS AND METHODS
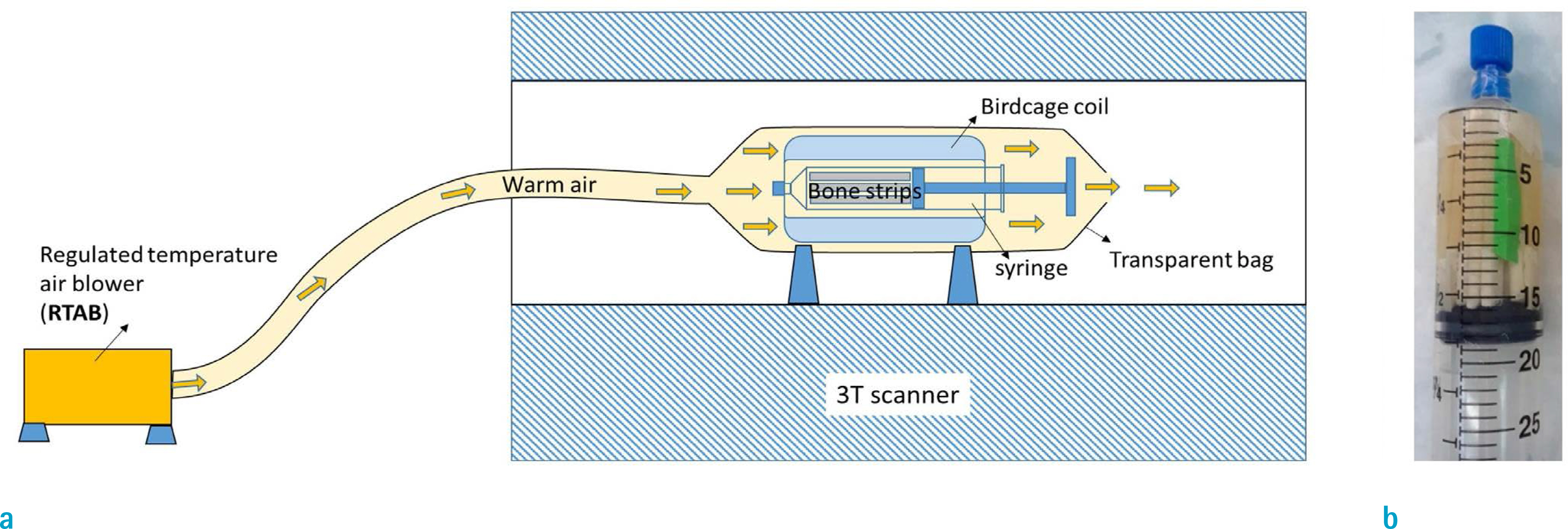
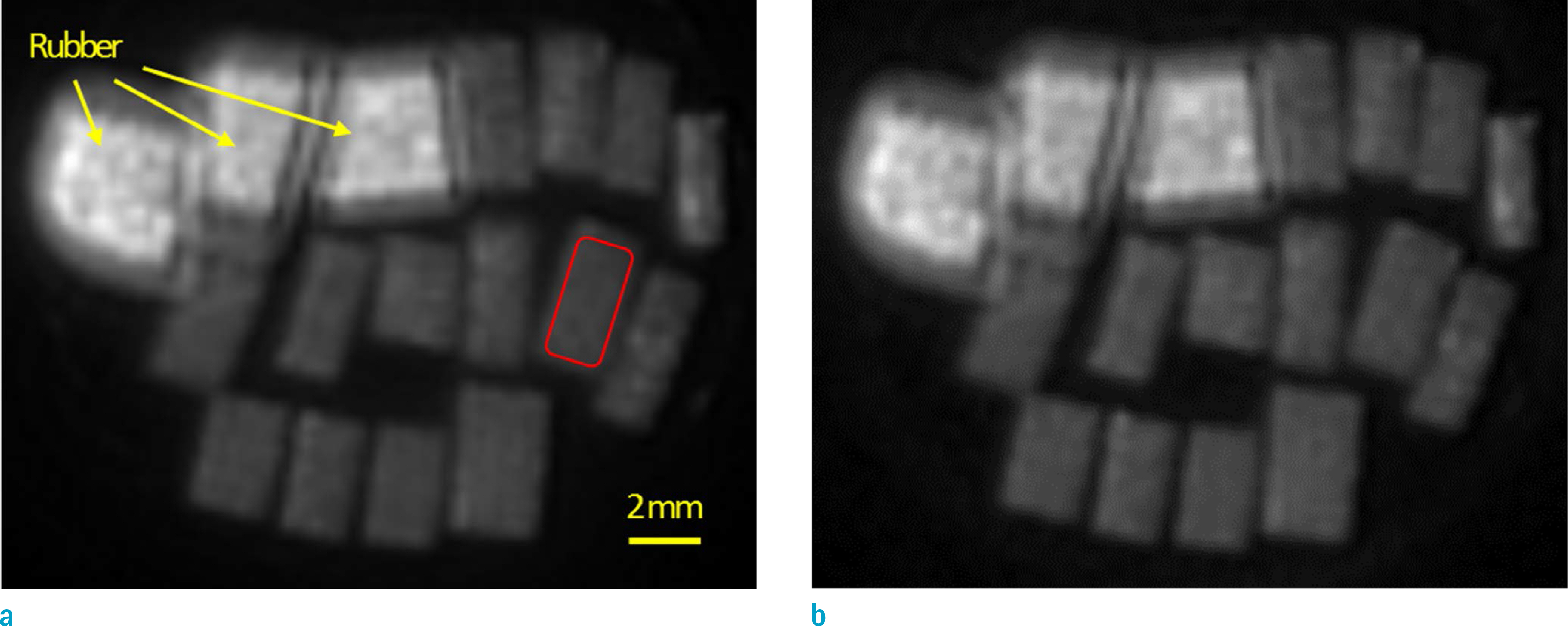
Cortical bone specimens from fourteen donors (63 ± 21 years old, 6 females and 8 males) were scanned on a 3T clinical scanner at body and room temperatures to perform T1, T2*, inversion recovery UTE (IR-UTE) T2* measurements, and two-pool magnetization transfer (MT) modeling.
RESULTS
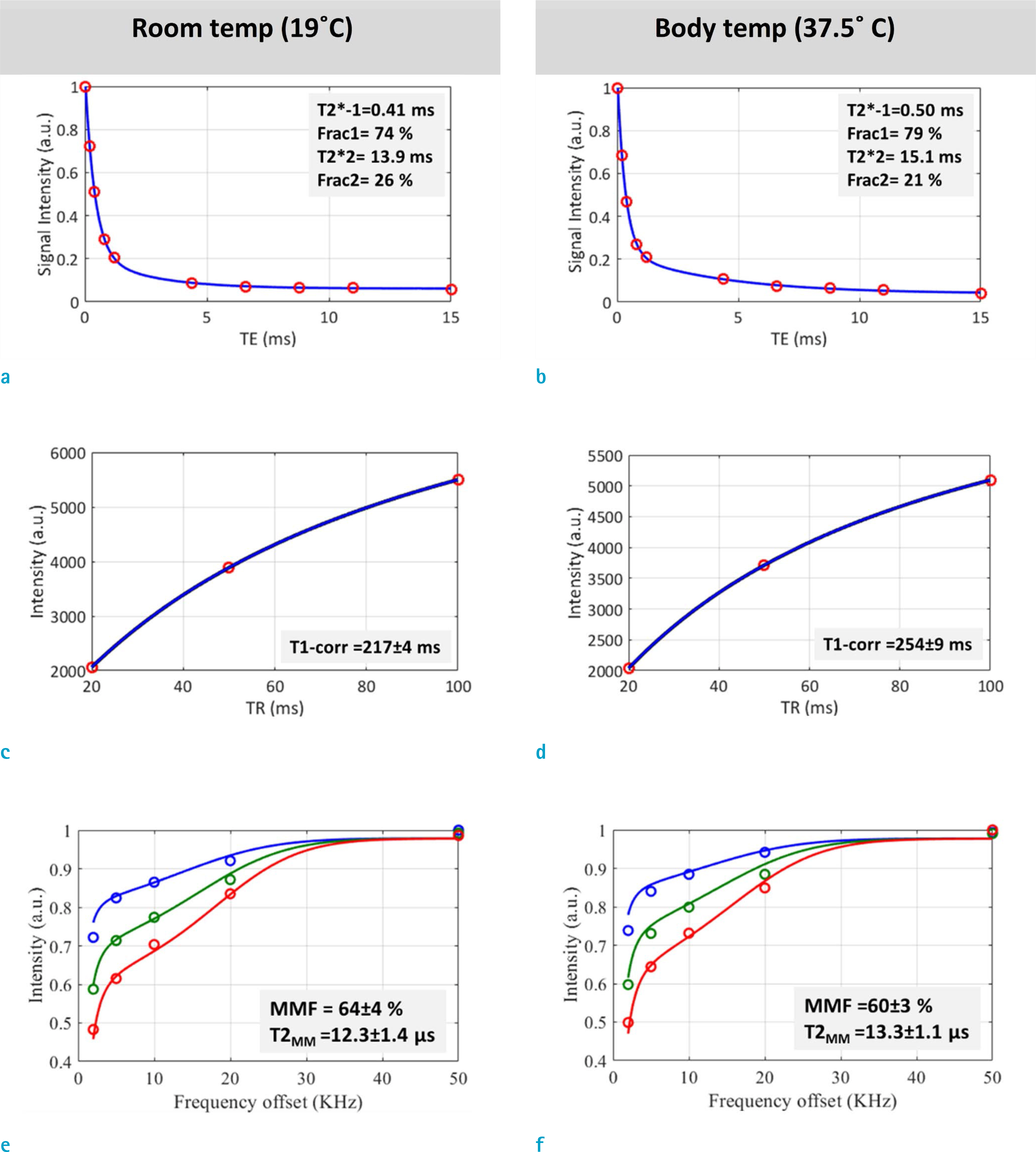
Single-component T2*, IR-T2*, short and long component T2*s from bi-component analysis, and T1 showed significantly higher values while the noted macromolecular fraction (MMF) from MT modeling showed significantly lower values at body temperature, as compared with room temperature. However, it is noted that the short component fraction (Frac1) showed higher values at body temperature.
CONCLUSION
This study highlights the need for careful consideration of the temperature effects on MRI measurements, before extending a conclusion from ex vivo studies on cortical bone specimens to clinical in vivo studies. It is noted that the increased relaxation times at higher temperature was most likely due to an increased molecular motion. The T1 increase for the studied human bone specimens was noted as being significantly higher than the previously reported values for bovine cortical bone. The prevailing discipline notes that the increased relaxation times of the bound water likely resulted in a lower signal loss during data acquisition, which led to the incidence of a higher Frac1 at body temperature.
MeSH Terms
Figure
Reference
-
References
1. Seifert AC, Wehrli FW. Solid-state quantitative (1)H and (31)P MRI of cortical bone in humans. Curr Osteoporos Rep. 2016; 14:77–86.
Article2. Granke M, Does MD, Nyman JS. The role of water compartments in the material properties of cortical bone. Calcif Tissue Int. 2015; 97:292–307.
Article3. Nyman JS, Ni Q, Nicolella DP, Wang X. Measurements of mobile and bound water by nuclear magnetic resonance correlate with mechanical properties of bone. Bone. 2008; 42:193–199.
Article4. Chang EY, Du J, Chung CB. UTE imaging in the musculoskeletal system. J Magn Reson Imaging. 2015; 41:870–883.
Article5. Du J, Bydder GM. Qualitative and quantitative ultrashort-TE MRI of cortical bone. NMR Biomed. 2013; 26:489–506.
Article6. Lee H, Zhao X, Song HK, Zhang R, Bartlett SP, Wehrli FW. Rapid dual-RF, dual-echo, 3D ultrashort echo time craniofacial imaging: a feasibility study. Magn Reson Med. 2019; 81:3007–3016.
Article7. Lu X, Jerban S, Wan L, et al. Three-dimensional ultrashort echo time imaging with tricomponent analysis for human cortical bone. Magn Reson Med. 2019; 82:348–355.
Article8. Wan L, Zhao W, Ma Y, et al. Fast quantitative 3D ultrashort echo time MRI of cortical bone using extended cones sampling. Magn Reson Med. 2019; 82:225–236.
Article9. Jerban S, Ma Y, Wan L, et al. Collagen proton fraction from ultrashort echo time magnetization transfer (UTEMT) MRI modelling correlates significantly with cortical bone porosity measured with micro-computed tomography (muCT). NMR Biomed. 2019; 32:e4045.10. Rajapakse CS, Bashoor-Zadeh M, Li C, Sun W, Wright AC, Wehrli FW. Volumetric cortical bone porosity assessment with MR imaging: validation and clinical feasibility. Radiology. 2015; 276:526–535.
Article11. Manhard MK, Nyman JS, Does MD. Advances in imaging approaches to fracture risk evaluation. Transl Res. 2017; 181:1–14.
Article12. Diaz E, Chung CB, Bae WC, et al. Ultrashort echo time spectroscopic imaging (UTESI): an efficient method for quantifying bound and free water. NMR Biomed. 2012; 25:161–168.
Article14. Bae WC, Chen PC, Chung CB, Masuda K, D'Lima D, Du J. Quantitative ultrashort echo time (UTE) MRI of human cortical bone: correlation with porosity and biomechanical properties. J Bone Miner Res. 2012; 27:848–857.
Article15. Granke M, Makowski AJ, Uppuganti S, Does MD, Nyman JS. Identifying novel clinical surrogates to assess human bone fracture toughness. J Bone Miner Res. 2015; 30:1290–1300.
Article16. Li C, Seifert AC, Rad HS, et al. Cortical bone water concentration: dependence of MR imaging measures on age and pore volume fraction. Radiology. 2014; 272:796–806.
Article17. Johnson EM, Vyas U, Ghanouni P, Pauly KB, Pauly JM. Improved cortical bone specificity in UTE MR imaging. Magn Reson Med. 2017; 77:684–695.
Article18. Chen J, Chang EY, Carl M, et al. Measurement of bound and pore water T1 relaxation times in cortical bone using three-dimensional ultrashort echo time cones sequences. Magn Reson Med. 2017; 77:2136–2145.
Article19. Ma YJ, Lu X, Carl M, et al. Accurate T1 mapping of short T2 tissues using a three-dimensional ultrashort echo time cones actual flip angle imaging-variable repetition time (3D UTE-Cones AFI-VTR) method. Magn Reson Med. 2018; 80:598–608.20. Du J, Diaz E, Carl M, Bae W, Chung CB, Bydder GM. Ultrashort echo time imaging with bicomponent analysis. Magn Reson Med. 2012; 67:645–649.
Article21. Chang EY, Bae WC, Shao H, et al. Ultrashort echo time magnetization transfer (UTE-MT) imaging of cortical bone. NMR Biomed. 2015; 28:873–880.
Article22. Ma YJ, Tadros A, Du J, Chang EY. Quantitative two-dimensional ultrashort echo time magnetization transfer (2D UTE-MT) imaging of cortical bone. Magn Reson Med. 2018; 79:1941–1949.
Article23. Ma YJ, Shao H, Du J, Chang EY. Ultrashort echo time magnetization transfer (UTE-MT) imaging and modeling: magic angle independent biomarkers of tissue properties. NMR Biomed. 2016; 29:1546–1552.
Article24. Jerban S, Ma Y, Nazaran A, et al. Detecting stress injury (fatigue fracture) in fibular cortical bone using quantitative ultrashort echo time-magnetization transfer (UTE-MT): an ex vivo study. NMR Biomed. 2018; 31:e3994.
Article25. Ozhinsky E, Han M, Bucknor M, Krug R, Rieke V. T2-based temperature monitoring in bone marrow for MR-guided focused ultrasound. J Ther Ultrasound. 2016; 4:26.
Article26. Han M, Scott SJ, Ozhinsky E, et al. Assessing temperature changes in cortical bone using variable flip-angle ultrashort echo-time MRI. AIP Conference Proceedings. 2017. (1821):060001.
Article27. Ramsay E, Mougenot C, Kazem M, Laetsch TW, Chopra R. Temperature-dependent MR signals in cortical bone: potential for monitoring temperature changes during high-intensity focused ultrasound treatment in bone. Magn Reson Med. 2015; 74:1095–1102.
Article28. Rieke V, Butts Pauly K. MR thermometry. J Magn Reson Imaging. 2008; 27:376–390.
Article29. Han M, Rieke V, Scott SJ, et al. Quantifying temperature-dependent T1 changes in cortical bone using ultrashort echo-time MRI. Magn Reson Med. 2015; 74:1548–1555.30. Ma YJ, Chang EY, Carl M, Du J. Quantitative magnetization transfer ultrashort echo time imaging using a time-efficient 3D multispoke Cones sequence. Magn Reson Med. 2018; 79:692–700.
Article31. Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magn Reson Med. 2006; 55:575–582.
Article32. Carl M, Bydder GM, Du J. UTE imaging with simultaneous water and fat signal suppression using a time-efficient multispoke inversion recovery pulse sequence. Magn Reson Med. 2016; 76:577–582.
Article33. Ma YJ, Zhu Y, Lu X, Carl M, Chang EY, Du J. Short T2 imaging using a 3D double adiabatic inversion recovery prepared ultrashort echo time cones (3D DIR-UTE-Cones) sequence. Magn Reson Med. 2018; 79:2555–2563.34. Biswas R, Bae W, Diaz E, et al. Ultrashort echo time (UTE) imaging with bicomponent analysis: bound and free water evaluation of bovine cortical bone subject to sequential drying. Bone. 2012; 50:749–755.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrashort Echo Time and Zero Echo Time MRI and Their Applications at High Magnetic Fields: A Literature Survey
- Depiction of the Periosteum Using Ultrashort Echo Time Pulse Sequence with Three-Dimensional Cone Trajectory and Histologic Correlation in a Porcine Model
- Body Temperature Change during Surgery and General Anesthesia
- Sleep and Temperature
- Influence of receiver bandwidth on MRI artifacts caused by orthodontic brackets composed of different alloys