J Korean Soc Spine Surg.
2019 Sep;26(3):84-93. 10.4184/jkss.2019.26.3.84.
Surgical Extent of Metastatic Spine Tumor Excision and Its Effects on Postoperative Ambulatory Function: Comparison of Extensive Wide versus Palliative Excision Surgery
- Affiliations
-
- 1Department of Orthopedic Surgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. boscoa@catholic.ac.kr
- 2Department of Orthopedic Surgery, Gangdong Kyung Hee Hospital, College of Medicine, The Kyung Hee University of Korea, Seoul, Korea.
- 3Department of Orthopedic Surgery, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea.
- 4Department of Orthopedic Surgery, Eunpyeong St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2459757
- DOI: http://doi.org/10.4184/jkss.2019.26.3.84
Abstract
- STUDY DESIGN: Retrospective study.
OBJECTIVES
To compare surgical outcomes such as the ambulatory period and survival according to different surgical excision tactics for metastatic spine tumors (MSTs). SUMMARY OF LITERATURE REVIEW: Surgical outcomes, such as pain relief and survival, in patients with MSTs have been reported in several studies, but the effects of differences in surgical extent on the ambulatory period have rarely been reported.
MATERIALS AND METHODS
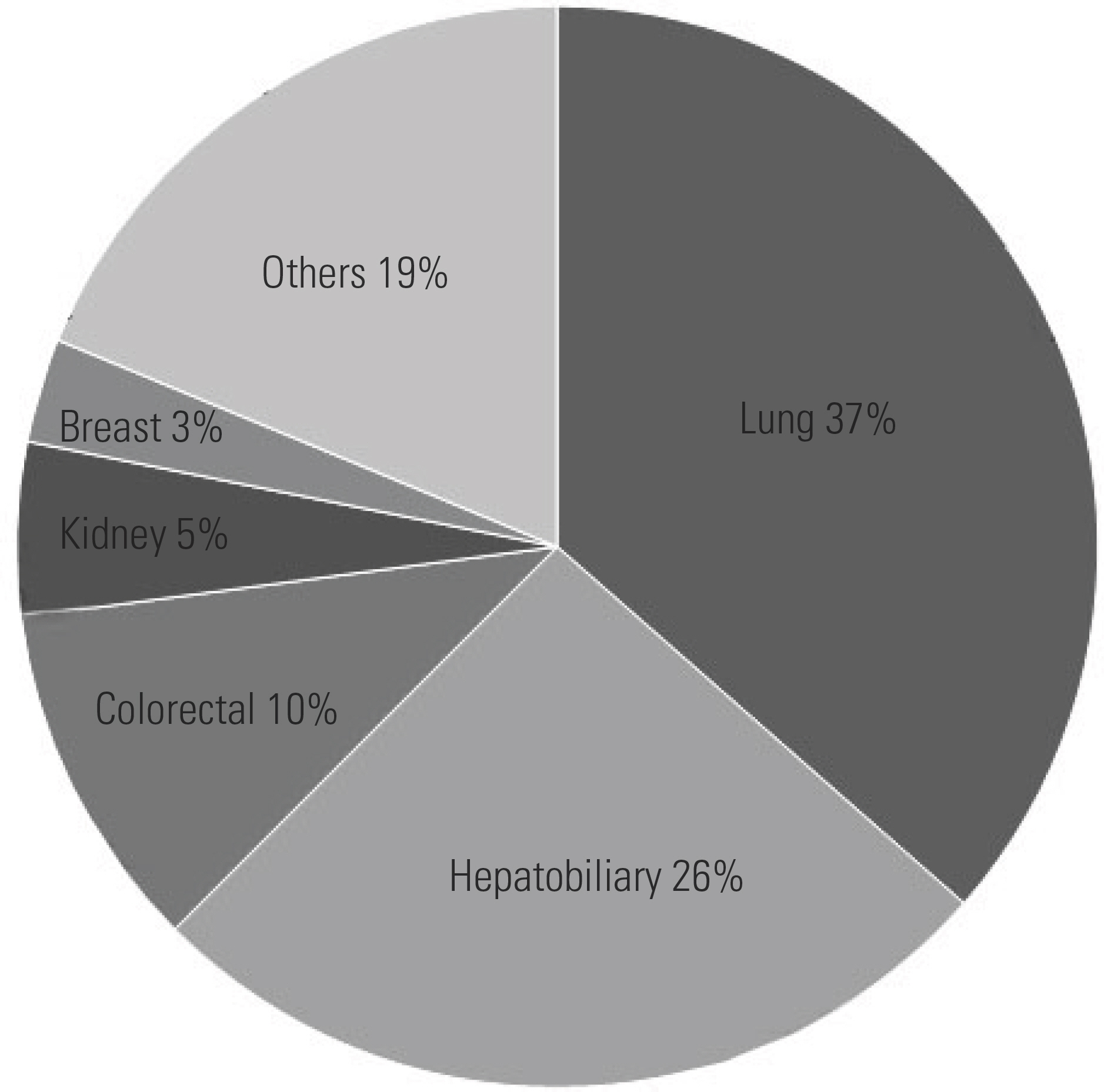
Ninety-six patients with MSTs who underwent palliative (n=60) or extensive wide excision (n=36) were included. Palliative excision was defined as partial removal of the tumor as an intralesional piecemeal procedure for decompression. Extensive wide excision was defined as a surgical attempt to remove the whole tumor at the index level as completely as possible. The primary outcome was the ambulatory period following surgery. Other demographic and radiographic parameters were analyzed to identify the risk factors for loss of ambulatory ability and survival. Perioperative complications were also assessed.
RESULTS
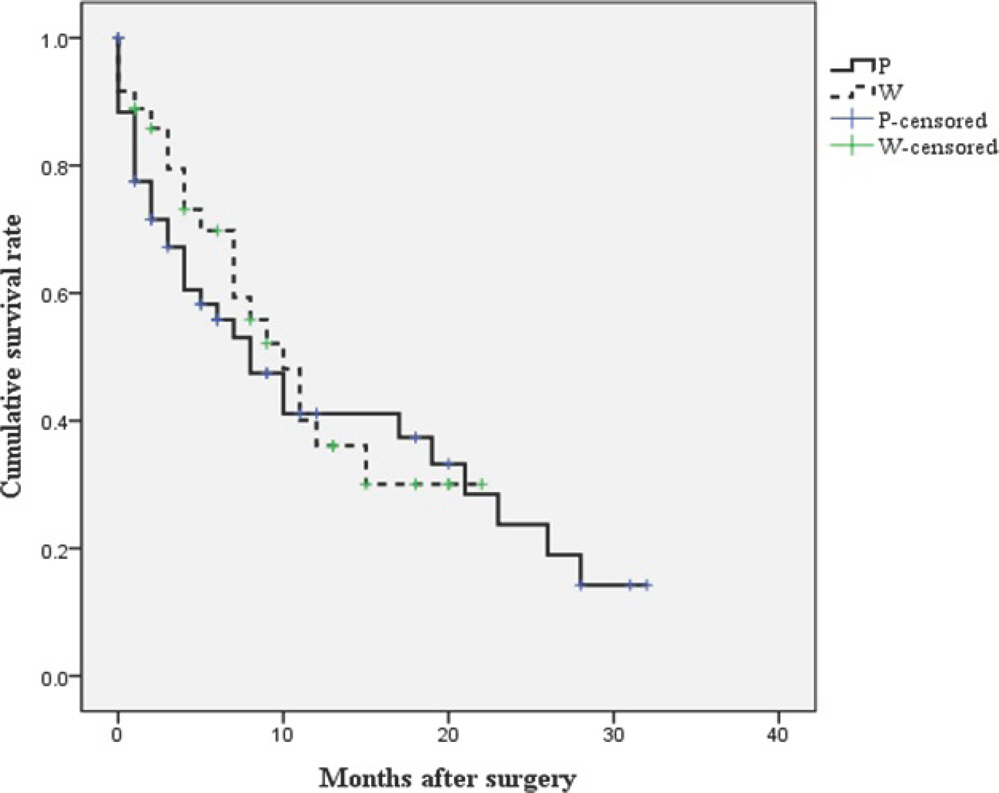
The mean postoperative ambulatory period was longer in the extensive wide excision group (average 14.8 months) than in the palliative excision group (average 11.7 months) (p=0.021). The survival rates were not significantly different between the two surgical excision groups (p=0.680). However, postoperative ambulatory status and major complications within 30 days postoperatively were significant prognostic factors for survival (p=0.003 and p=0.032, respectively).
CONCLUSIONS
The extent of surgical excision affected the ambulatory period, and the complication rates were similar, regardless of surgical excision tactics. A proper surgical strategy to achieve postoperative ambulatory ability and to reduce perioperative complications would have a favorable effect on survival.
Keyword
Figure
Reference
-
1. Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease. A review. J Neurosurg Spine. 2010 Jul; 13(1):E94–108. DOI: 10.3171/2010.3.spine09202.2. Sohn S, Kim J, Chung CK, et al. A nationwide epidemio-logical study of newly diagnosed spine metastasis in the adult Korean population. Spine J. 2016 Mar; 16(8):E937–45. DOI: 10.1016/j.spinee.2016.03.006.
Article3. Perrin RG, Laxton AW. Metastatic spine disease: epidemi-ology, pathophysiology, and evaluation of patients. Neurosurg Clin N Am. 2004 Sep; 15(4):E365–73. DOI: 10.1016/j.nec.2004.04.018.
Article4. Al-Qurainy R, Collis E. Metastatic spinal cord compression: diagnosis and management. BMJ (Clinical research ed). 2016 May; E353:i2539. DOI: 10.1136/bmj.i2539.
Article5. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet (London, England). 2005 Aug; 366(9486):E643–8. DOI: 10.1016/s0140-6736 (05)66954-1.
Article6. Choi D, Fox Z, Albert T, et al. Rapid improvements in pain and quality of life are sustained after surgery for spinal metastases in a large prospective cohort. British journal of neurosurgery. 2016 Feb; 30(3):E337–44. DOI: 10.3109/02688697.2015.1133802.
Article7. Ibrahim A, Crockard A, Antonietti P, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. Invited sub-mission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. Journal of neurosurgery Spine. 2008 Mar; 8(3):E271–8. DOI: 10.3171/spi/2008/8/3/271.8. Kakutani K, Sakai Y, Maeno K, et al. Prospective Cohort Study of Performance Status and Activities of Daily Living After Surgery for Spinal Metastasis. Clin Spine Surg. 2017 Oct; 30(8):E1026–32. DOI: 10.1097/bsd.0000000000000456.
Article9. Tang Y, Qu J, Wu J, et al. Effect of Surgery on Quality of Life of Patients with Spinal Metastasis from Non-Small-Cell Lung Cancer. The Journal of bone and joint surgery American volume. 2016 Mar; 98(5):396–402. DOI: 10.2106/jbjs.o.00629.
Article10. Fourney DR, Frangou EM, Ryken TC, et al. Spinal insta-bility neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011 Aug; 29(22):3072–7. DOI: 10.1200/jco.2010.34.3897.
Article11. Tokuhashi Y, Matsuzaki H, Oda H, at al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 2005 Oct; 30(19):218691. DOI: 10.1097/01.brs.0000180401.06919.a5.12. Tomita K, Kawahara N, Kobayashi T, at al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976). 2001 Feb; 26(3):298–306. DOI: 10.1097/00007632-200102010-00016.13. Bakar D, Tanenbaum JE, Phan K, et al. Decompression surgery for spinal metastases: a systematic review. Neurosurg focus. 2016 Aug; 41(2):E2. DOI: 10.3171/2016.6.fo-cus16166.
Article14. Choi D, Crockard A, Bunger C, et al. Review of metastatic spine tumour classification and indications for sur-gery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J. 2010 Feb; 19(2):215–22. DOI: 10.1007/s00586-009-1252-x.
Article15. Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972; 95(1):87–100. DOI: 10.1093/brain/95.1.87.
Article16. Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010 Sep; 13(3):324–8. DOI: 10.3171/2010.3.spine09459.
Article17. Ho JC, Tang C, Deegan BJ, et al. The use of spine ste-reotactic radiosurgery for oligometastatic disease. J Neurosurg Spine. 2016 Aug; 25(2):239–47. DOI: 10.3171/2016.1.spine151166.
Article18. Abrahm JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer: “all I care about is walking and living my life”. JAMA. 2008 Feb; 299(8):937–46. DOI: 10.1001/jama.299.8.937.19. Chaichana KL, Woodworth GF, Sciubba DM, et al. Pre-dictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery. 2008 Mar; 62(3):683–92. discussion 683-92. DOI: 10.1227/01.neu.0000317317.33365.15.
Article20. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001 Jul; 33(5):337–43. DOI: 10.3109/07853890109002087.21. de Ruiter GC, Nogarede CO, Wolfs JF, at al. Quality of life after different surgical procedures for the treatment of spinal metastases: results of a single-center prospective case series. Neurosurg focus. 2017 Jan; 42(1):E17. DOI: 10.3171/2016.6.focus16150.22. Kim DG, Ha JK, Hwang CJ, at al. Is 1-stage Posterior Corpectomy More Favorable Compared to Decompression with Fusion to Control Thoracic Cord Compression by Metastasis? Clin Spine Surg. 2017 Oct; 30(8):350–55. DOI: 10.1097/bsd.0000000000000267.23. Kim CH, Chung CK, Jahng TA, at al. Resumption of ambulatory status after surgery for nonambulatory patients with epidural spinal metastasis. Spine J. 2011 Oct; 11(11):E1015–23. DOI: 10.1016/j.spinee.2011.09.007.24. Park SJ, Lee CS, Chung SS. Surgical results of metastatic spinal cord compression (MSCC) from non-small cell lung cancer (NSCLC): analysis of functional outcome, survival time, and complication. Spine J. 2016 Mar; 16(3):322–8. DOI: 10.1016/j.spinee.2015.11.005.
Article25. Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine (Phila Pa 1976). 1997 May; 22(9):1036–44. DOI: 10.1097/00007632-199705010-00020.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Chondrosarcoma of the Hyoid Bone
- A Treatment of Recurrent Clear Cell Hidradenoma on the Neck: A Case Report
- Giant-cell Tumor of the Lumbar Spine: Case Report
- Study on the Comparison between Wide Excision and Mohs Micrographic Surgery for the Management of Dermatofibrosarcoma Protuberans: A Single Institution Experience
- Comparison of complete surgical excision and minimally invasive excision using COâ‚‚ laser for removal of epidermal cysts on the face