Chonnam Med J.
2018 Jan;54(1):48-54. 10.4068/cmj.2018.54.1.48.
Predictive Value of Procalcitonin for Infection and Survival in Adult Cardiogenic Shock Patients Treated with Extracorporeal Membrane Oxygenation
- Affiliations
-
- 1Department of Thoracic and Cardiovascular Surgery, Chonnam National University Hospital, Gwangju, Korea. isjeong1201@gmail.com, bsoh@jnu.ac.kr
- 2Department of Pediatrics, Chonnam National University Hospital, Gwangju, Korea.
- 3Research Institute of Medical Sciences, Chonnam National University, Gwangju, Korea.
- KMID: 2458585
- DOI: http://doi.org/10.4068/cmj.2018.54.1.48
Abstract
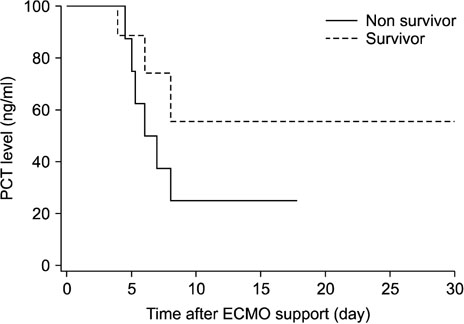
- Procalcitonin (PCT) is a predictive marker for the occurrence of bacterial infection and the decision to terminate antibiotic treatment in critically ill patients. An unusual increase in PCT, regardless of infection, has been observed during extracorporeal membrane oxygenation (ECMO) support. We evaluated trends and the predictive value of PCT levels in adult cardiogenic shock during treatment with ECMO. We reviewed the clinical records of 38 adult cardiogenic shock patients undergoing veno-arterial ECMO support between January 2014 and December 2016. The exclusion criteria were age < 18 years, pre-ECMO infection, and less than 48 hours of support. The mean patient age was 56.7±14.7 years and 12 (31.6%) patients were female. The mean duration of ECMO support was 9.0±7.6 days. The rates of successful ECMO weaning and survival to discharge were 55.3% (n=21) and 52.6% (n=20), respectively. There were 17 nosocomial infections in 16 (42.1%) patients. Peak PCT levels (mean 25.6±9.4 ng/mL) were reached within 48 hours after initiation of ECMO support and decreased to ≤5 ng/mL within one week. The change in PCT levels was not useful in predicting the occurrence of new nosocomial infections during the ECMO run. However, a PCT level >10 ng/mL during the first week of ECMO support was significantly associated with mortality (p < 0.01). The change in PCT level was not useful in predicting new infection during ECMO support. However, higher PCT levels within the first week of the ECMO run are associated with significantly higher mortality.
MeSH Terms
Figure
Reference
-
1. Cho HJ, Kim DW, Kim GS, Jeong IS. Anticoagulation therapy during extracorporeal membrane oxygenator support in pediatric patients. Chonnam Med J. 2017; 53:110–117.
Article2. Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P. Extracorporeal Life Support Organization Task Force on Infections. Extracorporeal Membrane Oxygenation. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. 2011; 12:277–281.
Article3. Sandkovsky U, Kalil AC, Florescu DF. The use and value of procalcitonin in solid organ transplantation. Clin Transplant. 2015; 29:689–696.
Article4. Cossé C, Sabbagh C, Carroni V, Galmiche A, Rebibo L, Regimbeau JM. Impact of a procalcitonin-based algorithm on the management of adhesion-related small bowel obstruction. J Visc Surg. 2017; 154:231–237.
Article5. Cosse C, Sabbagh C, Kamel S, Galmiche A, Regimbeau JM. Procalcitonin and intestinal ischemia: a review of the literature. World J Gastroenterol. 2014; 20:17773–17778.
Article6. Li YM, Liu XY. Serum levels of procalcitonin and high sensitivity C-reactive protein are associated with long-term mortality in acute ischemic stroke. J Neurol Sci. 2015; 352:68–73.
Article7. Rungatscher A, Merlini A, De Rita F, Lucchese G, Barozzi L, Faggian G, et al. Diagnosis of infection in paediatric veno-arterial cardiac extracorporeal membrane oxygenation: role of procalcitonin and C-reactive protein. Eur J Cardiothorac Surg. 2013; 43:1043–1049.
Article8. Tanaka D, Pitcher HT, Cavarocchi NC, Diehl JT, Hirose H. Can procalcitonin differentiate infection from systemic inflammatory reaction in patients on extracorporeal membrane oxygenation? J Heart Lung Transplant. 2014; 33:1186–1188.
Article9. Kim GS, Lee KS, Park CK, Kang SK, Kim DW, Oh SG, et al. Nosocomial Infection in Adult Patients Undergoing Veno-Arterial Extracorporeal Membrane Oxygenation. J Korean Med Sci. 2017; 32:593–598.
Article10. ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support. Extracorporeal Life Support Organization [Internet]. Ann Arbor: Extracorporeal Life Support Organization;c2017. cited 2017 Oct 6. Available from: https://www.elso.org.11. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017; 64:1509–1515.
Article12. Schuetz P, Briel M, Mueller B. Clinical outcomes associated with procalcitonin algorithms to guide antibiotic therapy in respiratory tract infections. JAMA. 2013; 309:717–718.
Article13. Schuetz P, Briel M, Christ-Crain M, Stolz D, Bouadma L, Wolff M, et al. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: an individual patient data meta-analysis. Clin Infect Dis. 2012; 55:651–662.
Article14. Sager R, Kutz A, Mueller B, Schuetz P. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017; 15:15.
Article15. Bloos F, Trips E, Nierhaus A, Briegel J, Heyland DK, Jaschinski U, et al. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Intern Med. 2016; 176:1266–1276.
Article16. Schuetz P, Daniels LB, Kulkarni P, Anker SD, Mueller B. Procalcitonin: a new biomarker for the cardiologist. Int J Cardiol. 2016; 223:390–397.
Article17. Nunez Lopez O, Cambiaso-Daniel J, Branski LK, Norbury WB, Herndon DN. Predicting and managing sepsis in burn patients: current perspectives. Ther Clin Risk Manag. 2017; 13:1107–1117.
Article18. Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013; 13:426–435.
Article19. Brunkhorst FM, Wegscheider K, Forycki ZF, Brunkhorst R. Procalcitonin for early diagnosis and differentiation of SIRS, sepsis, severe sepsis, and septic shock. Intensive Care Med. 2000; 26:Suppl 2. S148–S152.
Article20. Verduri A, Luppi F, D'Amico R, Balduzzi S, Vicini R, Liverani A, et al. Antibiotic treatment of severe exacerbations of chronic obstructive pulmonary disease with procalcitonin: a randomized noninferiority trial. PLoS One. 2015; 10:e0118241.
Article21. Prkno A, Wacker C, Brunkhorst FM, Schlattmann P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock--a systematic review and meta-analysis. Crit Care. 2013; 17:R291.22. Klingele M, Bomberg H, Schuster S, Schäfers HJ, Groesdonk HV. Prognostic value of procalcitonin in patients after elective cardiac surgery: a prospective cohort study. Ann Intensive Care. 2016; 6:116.
Article23. Liu H, Luo Z, Liu L, Yang XM, Zhuang YM, Zhang Y, et al. Early kinetics of procalcitonin in predicting surgical outcomes in type a aortic dissection patients. Chin Med J (Engl). 2017; 130:1175–1181.
Article24. Minami E, Ito S, Sugiura T, Fujita Y, Sasano H, Sobue K. Markedly elevated procalcitonin in early postoperative period in pediatric open heart surgery: a prospective cohort study. J Intensive Care. 2014; 2:38.
Article25. Beghetti M, Rimensberger PC, Kalangos A, Habre W, Gervaix A. Kinetics of procalcitonin, interleukin 6 and C-reactive protein after cardiopulmonary-bypass in children. Cardiol Young. 2003; 13:161–167.
Article26. Pieri M, Greco T, De Bonis M, Maj G, Fumagalli L, Zangrillo A, et al. Diagnosis of infection in patients undergoing extracorporeal membrane oxygenation: a case-control study. J Thorac Cardiovasc Surg. 2012; 143:1411–1416.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of rescuing a patient with acute cardiovascular instability from sudden and massive intraoperative pulmonary thromboembolism by extracorporeal membrane oxygenation
- Catastrophic catecholamine-induced cardiomyopathy rescued by extracorporeal membrane oxygenation in recurrent malignant pheochromocytoma
- Acute fulminant myocarditis following influenza vaccination requiring extracorporeal membrane oxygenation
- Mechanical Circulatory Support in the Cardiac Catheterization Laboratory for Cardiogenic Shock
- Erratum: Predictive Value of Procalcitonin for Infection and Survival in Adult Cardiogenic Shock Patients Treated with Extracorporeal Membrane Oxygenation