Korean J Radiol.
2016 Oct;17(5):725-733. 10.3348/kjr.2016.17.5.725.
Evaluation of the Differences of Myocardial Fibers between Acute and Chronic Myocardial Infarction: Application of Diffusion Tensor Magnetic Resonance Imaging in a Rhesus Monkey Model
- Affiliations
-
- 1Department of Radiology, West China Hospital, Sichuan University, Sichuan 610041, China. gaofabao_sub@163.com
- 2CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology of China, Beijing 100190, China.
- 3Department of Radiology, Beijing Jishuitan Hospital, 4th Clinical Medical College of Peking University, Beijing 100035, China.
- 4Department of Radiology, The First Affiliated Hospital, Chongqing Medical University, Chongqing 400016, China.
- 5Department of Radiology, The First Affiliated Hospital of Kunming Medical University, Yunnan 650032, China.
- 6Mallinckrodt Institute of Radiology, School of Medicine, Washington University, St. Louis, MO 63110, USA.
- KMID: 2458064
- DOI: http://doi.org/10.3348/kjr.2016.17.5.725
Abstract
OBJECTIVE
To understand microstructural changes after myocardial infarction (MI), we evaluated myocardial fibers of rhesus monkeys during acute or chronic MI, and identified the differences of myocardial fibers between acute and chronic MI.
MATERIALS AND METHODS
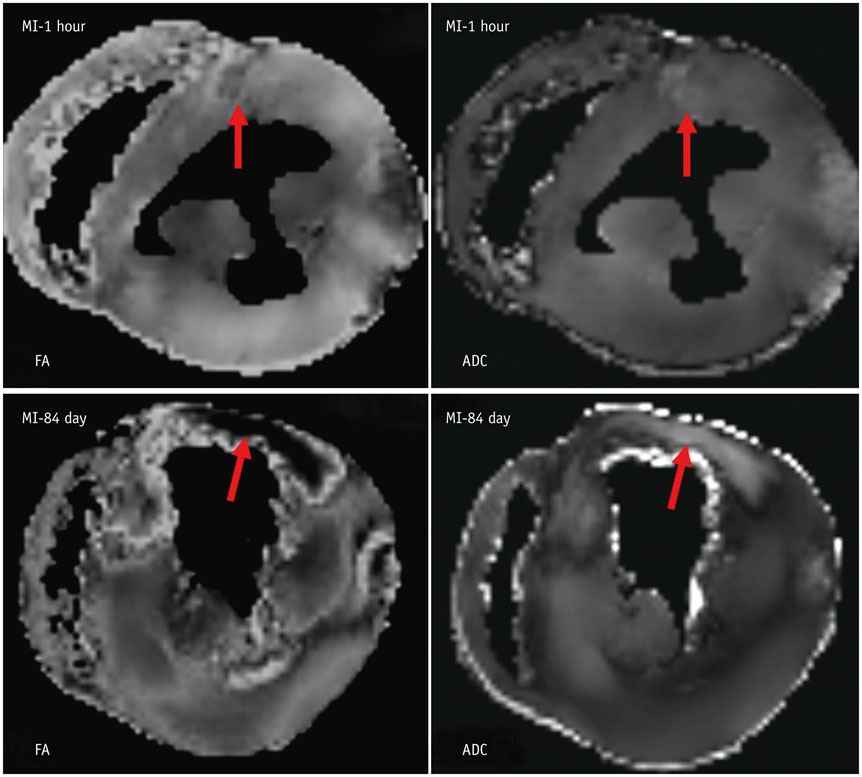
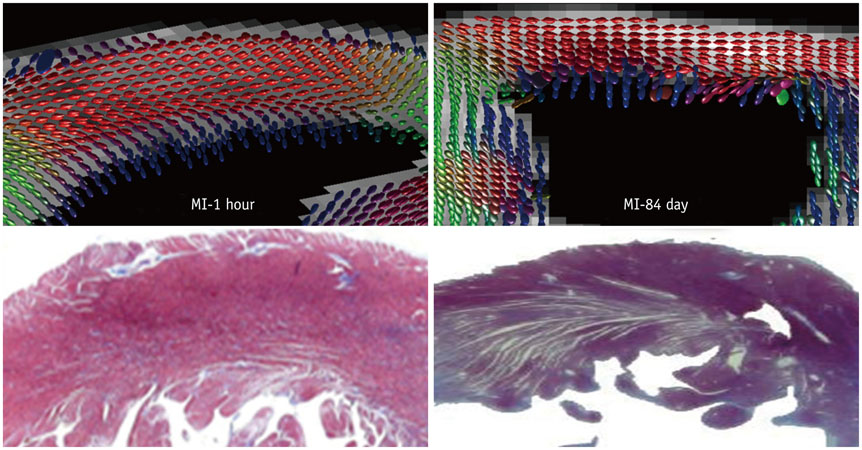
Six fixed hearts of rhesus monkeys with left anterior descending coronary artery ligation for 1 hour or 84 days were scanned by diffusion tensor magnetic resonance imaging (MRI) to measure apparent diffusion coefficient (ADC), fractional anisotropy (FA) and helix angle (HA).
RESULTS
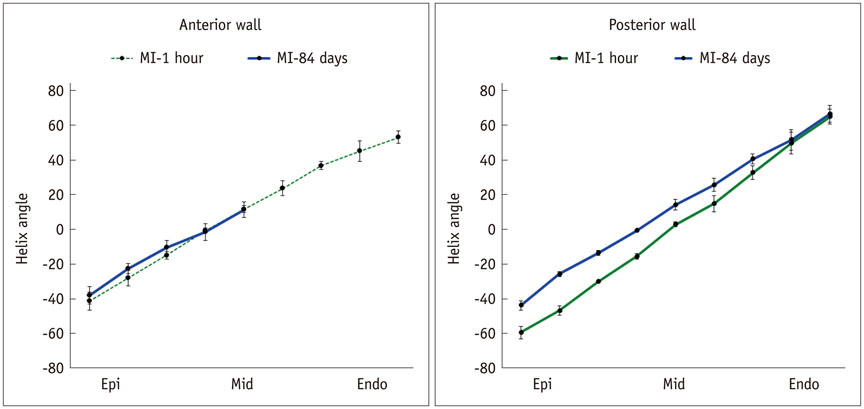
Comparing with acute MI monkeys (FA: 0.59 ± 0.02; ADC: 5.0 ± 0.6 × 10(-4) mm2/s; HA: 94.5 ± 4.4°), chronic MI monkeys showed remarkably decreased FA value (0.26 ± 0.03), increased ADC value (7.8 ± 0.8 × 10(-4) mm2/s), decreased HA transmural range (49.5 ± 4.6°) and serious defects on endocardium in infarcted regions. The HA in infarcted regions shifted to more components of negative left-handed helix in chronic MI monkeys (-38.3 ± 5.0°-11.2 ± 4.3°) than in acute MI monkeys (-41.4 ± 5.1°-53.1 ± 3.7°), but the HA in remote regions shifted to more components of positive right-handed helix in chronic MI monkeys (-43.8 ± 2.7°-66.5 ± 4.9°) than in acute MI monkeys (-59.5 ± 3.4°-64.9 ± 4.3°).
CONCLUSION
Diffusion tensor MRI method helps to quantify differences of mechanical microstructure and water diffusion of myocardial fibers between acute and chronic MI monkey's models.
Keyword
Figure
Cited by 1 articles
-
Rise of the Visible Monkey: Sectioned Images of Rhesus Monkey
Beom Sun Chung, Chang-Yeop Jeon, Jae-Won Huh, Kang-Jin Jeong, Donghwan Har, Kyu-Sung Kwack, Jin Seo Park
J Korean Med Sci. 2019;34(8):. doi: 10.3346/jkms.2019.34.e66.
Reference
-
1. Vadakkumpadan F, Arevalo H, Trayanova NA. Patient-specific modeling of the heart: estimation of ventricular fiber orientations. J Vis Exp. 2013; Jan. 8. [Epub]. DOI: 10.3791/50125.2. Lakshmanan R, Krishnan UM, Sethuraman S. Living cardiac patch: the elixir for cardiac regeneration. Expert Opin Biol Ther. 2012; 12:1623–1640.3. Wang Y, Haynor DR, Kim Y. An investigation of the importance of myocardial anisotropy in finite-element modeling of the heart: methodology and application to the estimation of defibrillation efficacy. IEEE Trans Biomed Eng. 2001; 48:1377–1389.4. Niederer SA, Buist ML, Pullan AJ, Smith NP. Computing work in the ischemic heart. Conf Proc IEEE Eng Med Biol Soc. 2004; 5:3646–3649.5. Vetter FJ, McCulloch AD. Three-dimensional analysis of regional cardiac function: a model of rabbit ventricular anatomy. Prog Biophys Mol Biol. 1998; 69:157–183.6. Wu MT, Tseng WY, Su MY, Liu CP, Chiou KR, Wedeen VJ, et al. Diffusion tensor magnetic resonance imaging mapping the fiber architecture remodeling in human myocardium after infarction: correlation with viability and wall motion. Circulation. 2006; 114:1036–1045.7. Wu MT, Su MY, Huang YL, Chiou KR, Yang P, Pan HB, et al. correlation with left ventricular structure and function. Circ Cardiovasc Imaging. 2000; 2:32–40.8. Peyrat JM, Sermesant M, Pennec X, Delingette H, Xu C, McVeigh ER, et al. Statistical comparison of cardiac fibre architectures. In : In : Sachse FB, Seemann G, editors. Functional imaging and modeling of the heart: 4th International Conference; June 7-9, 2007; Salt Lake City, UT, USA. Lecture notes in computer science, Vol 4466. Berlin, Heidelberg: Springer Berlin Heidelberg;2007. p. 413–423.9. Jiang Y, Guccione JM, Ratcliffe MB, Hsu EW. Transmural heterogeneity of diffusion anisotropy in the sheep myocardium characterized by MR diffusion tensor imaging. Am J Physiol Heart Circ Physiol. 2007; 293:H2377–H2384.10. Healy LJ, Jiang Y, Hsu EW. Quantitative comparison of myocardial fiber structure between mice, rabbit, and sheep using diffusion tensor cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011; 13:74.11. Mekkaoui C, Huang S, Chen HH, Dai G, Reese TG, Kostis WJ, et al. Fiber architecture in remodeled myocardium revealed with a quantitative diffusion CMR tractography framework and histological validation. J Cardiovasc Magn Reson. 2012; 14:70.12. Chen J, Song SK, Liu W, McLean M, Allen JS, Tan J, et al. Remodeling of cardiac fiber structure after infarction in rats quantified with diffusion tensor MRI. Am J Physiol Heart Circ Physiol. 2003; 285:H946–H954.13. Sosnovik DE, Wang R, Dai G, Wang T, Aikawa E, Novikov M, et al. Diffusion spectrum MRI tractography reveals the presence of a complex network of residual myofibers in infarcted myocardium. Circ Cardiovasc Imaging. 2009; 2:206–212.14. Hooks DA, Trew ML, Caldwell BJ, Sands GB, LeGrice IJ, Smaill BH. Laminar arrangement of ventricular myocytes influences electrical behavior of the heart. Circ Res. 2007; 101:e103–e112.15. Desai KV, Laine GA, Stewart RH, Cox CS Jr, Quick CM, Allen SJ, et al. Mechanics of the left ventricular myocardial interstitium: effects of acute and chronic myocardial edema. Am J Physiol Heart Circ Physiol. 2008; 294:H2428–H2434.16. Yang P, Han P, Hou J, Zhang L, Song H, Xie Y, et al. Electrocardiographic characterization of rhesus monkey model of ischemic myocardial infarction induced by left anterior descending artery ligation. Cardiovasc Toxicol. 2011; 11:365–372.17. Mohammadi S, Freund P, Feiweier T, Curt A, Weiskopf N. The impact of post-processing on spinal cord diffusion tensor imaging. Neuroimage. 2013; 70:377–385.18. Savadjiev P, Strijkers GJ, Bakermans AJ, Piuze E, Zucker SW, Siddiqi K. Heart wall myofibers are arranged in minimal surfaces to optimize organ function. Proc Natl Acad Sci U S A. 2012; 109:9248–9253.19. Geerts L, Bovendeerd P, Nicolay K, Arts T. Characterization of the normal cardiac myofiber field in goat measured with MR-diffusion tensor imaging. Am J Physiol Heart Circ Physiol. 2002; 283:H139–H145.20. Holmes AA, Scollan DF, Winslow RL. Direct histological validation of diffusion tensor MRI in formaldehyde-fixed myocardium. Magn Reson Med. 2000; 44:157–161.21. Wu Y, Zhang LJ, Zou C, Tse HF, Wu EX. Transmural heterogeneity of left ventricular myocardium remodeling in postinfarct porcine model revealed by MR diffusion tensor imaging. J Magn Reson Imaging. 2011; 34:43–49.22. Kuntz ID Jr, Brassfield TS, Law GD, Purcell GV. Hydration of macromolecules. Science. 1969; 163:1329–1331.23. Kloner RA, Rude RE, Carlson N, Maroko PR, DeBoer LW, Braunwald E. Ultrastructural evidence of microvascular damage and myocardial cell injury after coronary artery occlusion: which comes first. Circulation. 1980; 62:945–952.24. Barmpoutis A, Vemuri BC. A unified framework for estimating diffusion tensors of any order with symmetric positive-definite constraints. Proc IEEE Int Symp Biomed Imaging. 2010; 1385–1388.25. Helm PA, Younes L, Beg MF, Ennis DB, Leclercq C, Faris OP, et al. Evidence of structural remodeling in the dyssynchronous failing heart. Circ Res. 2006; 98:125–132.26. Tseng WY, Dou J, Reese TG, Wedeen VJ. Imaging myocardial fiber disarray and intramural strain hypokinesis in hypertrophic cardiomyopathy with MRI. J Magn Reson Imaging. 2006; 23:1–8.27. Ripplinger CM, Li W, Hadley J, Chen J, Rothenberg F, Lombardi R, et al. Enhanced transmural fiber rotation and connexin 43 heterogeneity are associated with an increased upper limit of vulnerability in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circ Res. 2007; 101:1049–1057.28. Helm PA, Tseng HJ, Younes L, McVeigh ER, Winslow RL. Ex vivo 3D diffusion tensor imaging and quantification of cardiac laminar structure. Magn Reson Med. 2005; 54:850–859.29. Lombaert H, Peyrat JM, Croisille P, Rapacchi S, Fanton L, Cheriet F, et al. Human atlas of the cardiac fiber architecture: study on a healthy population. IEEE Trans Med Imaging. 2012; 31:1436–1447.30. Toussaint N, Sermesant M, Stoeck CT, Kozerke S, Batchelor PG. In vivo human 3D cardiac fibre architecture: reconstruction using curvilinear interpolation of diffusion tensor images. Med Image Comput Comput Assist Interv. 2010; 13(Pt 1):418–425.31. Rohmer D, Sitek A, Gullberg GT. Reconstruction and visualization of fiber and laminar structure in the normal human heart from ex vivo diffusion tensor magnetic resonance imaging (DTMRI) data. Invest Radiol. 2007; 42:777–789.32. Benson AP, Bernus O, Dierckx H, Gilbert SH, Greenwood JP, Holden AV, et al. Construction and validation of anisotropic and orthotropic ventricular geometries for quantitative predictive cardiac electrophysiology. Interface Focus. 2011; 1:101–116.33. Vadakkumpadan F, Arevalo H, Prassl AJ, Chen J, Kickinger F, Kohl P, et al. Image-based models of cardiac structure in health and disease. Wiley Interdiscip Rev Syst Biol Med. 2010; 2:489–506.34. Wu EX, Wu Y, Nicholls JM, Wang J, Liao S, Zhu S, et al. MR diffusion tensor imaging study of postinfarct myocardium structural remodeling in a porcine model. Magn Reson Med. 2007; 58:687–695.35. Liu W, Ashford MW, Chen J, Watkins MP, Williams TA, Wickline SA, et al. MR tagging demonstrates quantitative differences in regional ventricular wall motion in mice, rats, and men. Am J Physiol Heart Circ Physiol. 2006; 291:H2515–H2521.36. Guilfoyle DN, Helpern JA, Lim KO. Diffusion tensor imaging in fixed brain tissue at 7.0 T. NMR Biomed. 2003; 16:77–78.37. Sun SW, Neil JJ, Liang HF, He YY, Schmidt RE, Hsu CY, et al. Formalin fixation alters water diffusion coefficient magnitude but not anisotropy in infarcted brain. Magn Reson Med. 2005; 53:1447–1451.38. Aggarwal M, Zhang J, Pletnikova O, Crain B, Troncoso J, Mori S. Feasibility of creating a high-resolution 3D diffusion tensor imaging based atlas of the human brainstem: a case study at 11.7 T. Neuroimage. 2013; 74:117–112.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advanced Cardiac MR Imaging for Myocardial Characterization and Quantification: T1 Mapping
- Differentiation of Acute Myocardial Infarction from Chronic Myocardial Scar with MRI
- Improving Delineation of the Corticospinal Tract in the Monkey Brain Scanned With Conventional Diffusion Tensor Imaging by Using a Compressed Sensing Based Algorithm
- High-Resolution Diffusion Tensor MR Imaging for Evaluating Myocardial Anisotropy and Fiber Tracking at 3T: the Effect of the Number of Diffusion-Sensitizing Gradient Directions
- Principle and Experiments in Diffusion Tensor Imaging