Ann Dermatol.
2018 Aug;30(4):409-416. 10.5021/ad.2018.30.4.409.
Effect of Evening Primrose Oil on Korean Patients With Mild Atopic Dermatitis: A Randomized, Double-Blinded, Placebo-Controlled Clinical Study
- Affiliations
-
- 1Department of Dermatology, Kangnam Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea. dermap@daum.net
- KMID: 2457517
- DOI: http://doi.org/10.5021/ad.2018.30.4.409
Abstract
- BACKGROUND
Atopic dermatitis (AD) is related to a deficiency of delta-6-desaturase, an enzyme responsible for converting linoleic acid to gamma-linolenic acid (GLA). Evening primrose oil (EPO) as a source of GLA has been of interest in the management of AD.
OBJECTIVE
The aim of this randomized, double-blinded, placebo-controlled clinical study is to evaluate the efficacy and safety of EPO in Korean patients with AD.
METHODS
Fifty mild AD patients with an Eczema Area Severity Index (EASI) score of 10 or less were enrolled and randomly divided into two groups. The first group received an oval unmarked capsule containing 450 mg of EPO (40 mg of GLA) per capsule, while placebo capsules identical in appearance and containing 450 mg of soybean oil were given to the other group. Treatment continued for a period of four months. EASI scores, transepidermal water loss (TEWL), and skin hydration were evaluated in all the AD patients at the baseline, and in months 1, 2, 3, and 4 of the study.
RESULTS
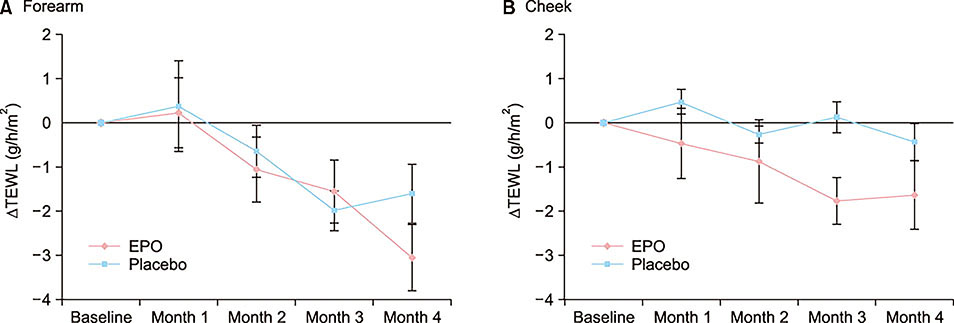
At the end of month 4, the patients of the EPO group showed a significant improvement in the EASI score (p=0.040), whereas the patients of the placebo group did not. There was a significant difference in the EASI score between the EPO and placebo groups (p=0.010). Although not statistically significant, the TEWL and skin hydration also slightly improved in the EPO patients group.
CONCLUSION
We suggest that EPO is a safe and effective medicine for Korean patients with mild AD.
MeSH Terms
Figure
Cited by 1 articles
-
Relation of polyunsaturated fatty acid, n-3 fatty acid and n-6 fatty acid intakes and atopic dermatitis in the 9 ~ 11 year old children: KNHANES 2013 ~ 2015
Ji-Myung Kim
J Nutr Health. 2019;52(1):47-57. doi: 10.4163/jnh.2019.52.1.47.
Reference
-
1. Bieber T. Atopic dermatitis. Ann Dermatol. 2010; 22:125–137.
Article2. Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009; 129:1892–1908.
Article3. McPherson T. Current Understanding in Pathogenesis of Atopic Dermatitis. Indian J Dermatol. 2016; 61:649–655.
Article4. Horrobin DF. Essential fatty acid metabolism and its modification in atopic eczema. Am J Clin Nutr. 2000; 71:1 Suppl. 367S–372S.
Article5. Wright S. Essential fatty acids and the skin. Br J Dermatol. 1991; 125:503–515.
Article6. Manku MS, Horrobin DF, Morse N, Kyte V, Jenkins K, Wright S, et al. Reduced levels of prostaglandin precursors in the blood of atopic patients: defective delta-6-desaturase function as a biochemical basis for atopy. Prostaglandins Leukot Med. 1982; 9:615–628.
Article7. Kerscher MJ, Korting HC. Treatment of atopic eczema with evening primrose oil: rationale and clinical results. Clin Investig. 1992; 70:167–171.
Article8. Simon D, Eng PA, Borelli S, Kägi R, Zimmermann C, Zahner C, et al. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv Ther. 2014; 31:180–188.
Article9. Senapati S, Banerjee S, Gangopadhyay DN. Evening primrose oil is effective in atopic dermatitis: a randomized placebo-controlled trial. Indian J Dermatol Venereol Leprol. 2008; 74:447–452.
Article10. Morse NL, Clough PM. A meta-analysis of randomized, placebo-controlled clinical trials of Efamol evening primrose oil in atopic eczema. Where do we go from here in light of more recent discoveries? Curr Pharm Biotechnol. 2006; 7:503–524.
Article11. Bamford JT, Ray S, Musekiwa A, van Gool C, Humphreys R, Ernst E. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev. 2013; (4):CD004416.
Article12. Lee JH, Kim KH, Kim MN, Kim JW, Ro YS, Park YL, et al. Report from ADRG: the treatment guideline of Korean atopic dermatitis. Korean J Dermatol. 2006; 44:907–913.13. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venerol (Stockh). 1980; 92:44–47.14. Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001; 10:11–18.
Article15. Eskin NAM. Borage and evening primrose oil. Eur J Lipid Sci Tech. 2008; 110:651–654.
Article16. Vieira BL, Lim NR, Lohman ME, Lio PA. Complementary and Alternative Medicine for Atopic Dermatitis: An Evidence-Based Review. Am J Clin Dermatol. 2016; 17:557–581.
Article17. Muggli R. Systemic evening primrose oil improves the biophysical skin parameters of healthy adults. Int J Cosmet Sci. 2005; 27:243–249.
Article18. Wertz PW, Swartzendruber DC, Abraham W, Madison KC, Downing DT. Essential fatty acids and epidermal integrity. Arch Dermatol. 1987; 123:1381–1384.
Article19. McCusker MM, Grant-Kels JM. Healing fats of the skin: the structural and immunologic roles of the omega-6 and omega-3 fatty acids. Clin Dermatol. 2010; 28:440–451.
Article20. Belch JJ, Hill A. Evening primrose oil and borage oil in rheumatologic conditions. Am J Clin Nutr. 2000; 71:1 Suppl. 352S–356S.
Article21. Manku MS, Horrobin DF, Morse NL, Wright S, Burton JL. Essential fatty acids in the plasma phospholipids of patients with atopic eczema. Br J Dermatol. 1984; 110:643–648.
Article22. Bamford JT, Gibson RW, Renier CM. Atopic eczema unresponsive to evening primrose oil (linoleic and gammalinolenic acids). J Am Acad Dermatol. 1985; 13:959–965.
Article23. Berth-Jones J, Graham-Brown RA. Placebo-controlled trial of essential fatty acid supplementation in atopic dermatitis. Lancet. 1993; 341:1557–1560.
Article24. Bordoni A, Biagi PL, Masi M, Ricci G, Fanelli C, Patrizi A, et al. Evening primrose oil (Efamol) in the treatment of children with atopic eczema. Drugs Exp Clin Res. 1988; 14:291–297.25. Schalin-Karrila M, Mattila L, Jansen CT, Uotila P. Evening primrose oil in the treatment of atopic eczema: effect on clinical status, plasma phospholipid fatty acids and circulating blood prostaglandins. Br J Dermatol. 1987; 117:11–19.
Article26. Humphreys F, Symons JA, Brown HK, Duff GW, Hunter JAA. The effects of gamolenic acid on adult atopic eczema and premenstrual exacerbation of eczema. Eur J Dermatol. 1994; 4:598–603.27. Yoon S, Lee J, Lee S. The therapeutic effect of evening primrose oil in atopic dermatitis patients with dry scaly skin lesions is associated with the normalization of serum gamma-interferon levels. Skin Pharmacol Appl Skin Physiol. 2002; 15:20–25.
Article28. Chung BY, Kim JH, Cho SI, Ahn IS, Kim HO, Park CW, et al. Dose-dependent effects of evening primrose oil in children and adolescents with atopic dermatitis. Ann Dermatol. 2013; 25:285–291.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dose-Dependent Effects of Evening Primrose Oil in Children and Adolescents with Atopic Dermatitis
- Effect of Evening Primrose Oil on Postmenopausal Psychological Symptoms: A Triple-Blind Randomized Clinical Trial
- The Suppressive Effect of Evening Primrose Oil on Murine Contact Sensitivity
- The Effect of Evening Primrose Oil Capsule on Hot Flashes and Night Sweats in Postmenopausal Women: A Single-Blind Randomized Controlled Trial
- Evening Primrose (Oenothera biennis) Oil in Management of Female Ailments