Cancer Res Treat.
2016 Apr;48(2):499-507. 10.4143/crt.2015.089.
Circulating Plasma Biomarkers for TSU-68, an Oral Antiangiogenic Agent, in Patients with Metastatic Breast Cancer
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sbkim3@amc.seoul.kr
- 2Center for Breast Cancer, National Cancer Center, Goyang, Korea.
- 3Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 4Division of Hematology-Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 6Department of Medicine, Keimyung University Dongsan Medical Center, Daegu, Korea.
- 7Department of Hematology and Oncology, Ajou University School of Medicine, Suwon, Korea.
- 8Department of Internal Medicine, Soonchunhyang University Hospital, Soonchunhyang University College of Medicine, Seoul, Korea.
- 9Department of Medical Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2454326
- DOI: http://doi.org/10.4143/crt.2015.089
Abstract
- PURPOSE
This study analyzed the role of plasma biomarkers for TSU-68 in a previous phase II trial comparing TSU-68 plus docetaxel and docetaxel alone in patients with metastatic breast cancer.
MATERIALS AND METHODS
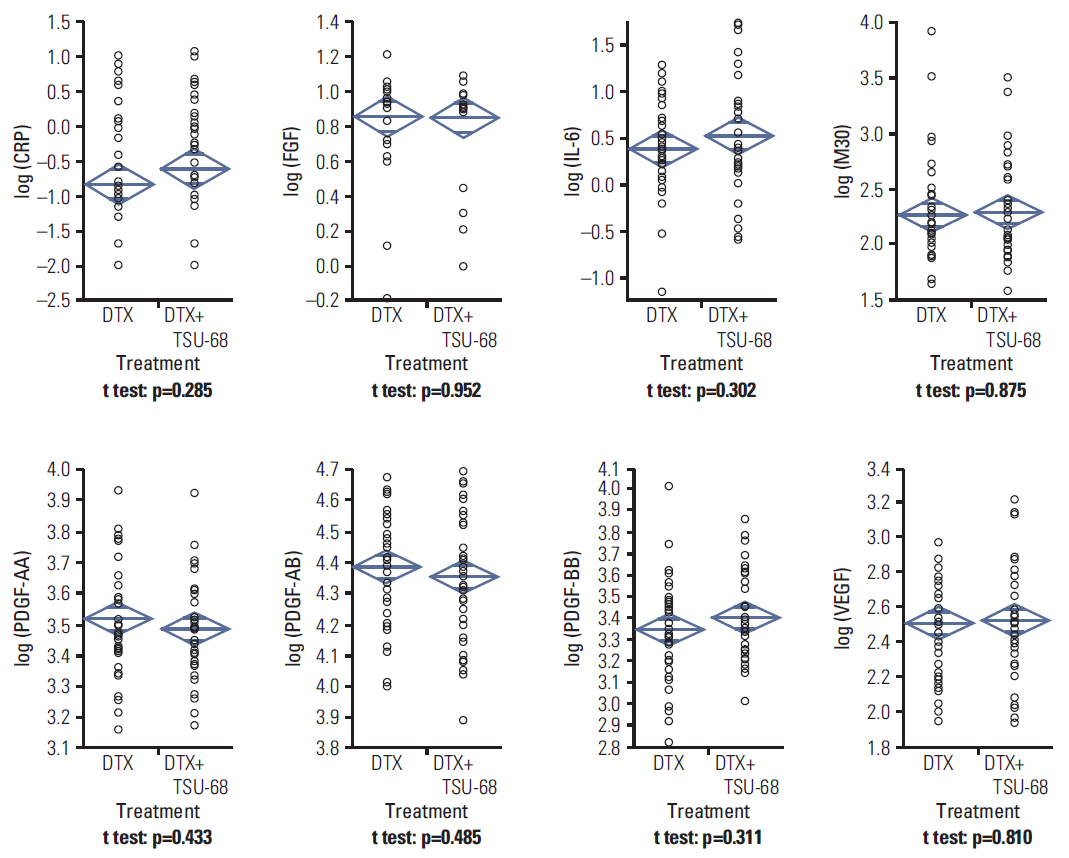
A total of 77 patients were eligible for this study (38 in the TSU-68 plus docetaxel arm and 39 in the docetaxel alone arm). Blood samples were collected prior to the start of each cycle, and vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF)-AA, -AB, -BB, fibroblast growth factor, M30, C-reactive protein (CRP), and interleukin 6 (IL-6) levels were measured using enzyme linked immunosorbent assay. The primary endpoint was progression-free survival (PFS).
RESULTS
In patients with baseline PDGF-AA ≥ median, median PFS was significantly worse in the TSU-68 plus docetaxel group than in the docetaxel alone group (5.4 months vs. 13.7 months, p=0.049), while a trend toward a PFS benefit was observed in those with baseline PDGF-AA < median (9.7 months vs. 4.0 months, p=0.18; p for interaction=0.03). In the TSU-68 plus docetaxel group, PFS showed significant association with fold changes in CRP (p=0.001), IL-6 (p < .001), PDGF-BB (p=0.02), and VEGF (p=0.047) following the first treatment cycle.
CONCLUSION
Baseline PDGF-AA levels and dynamics of VEGF, PDGF-BB, CRP, and IL-6 levels were predictive for the efficacy of TSU-68.
Keyword
MeSH Terms
-
Arm
Biomarkers*
Breast Neoplasms*
Breast*
C-Reactive Protein
Disease-Free Survival
Enzyme-Linked Immunosorbent Assay
Fibroblast Growth Factors
Humans
Interleukin-6
Pharmacology
Plasma*
Platelet-Derived Growth Factor
Vascular Endothelial Growth Factor A
Biomarkers
C-Reactive Protein
Fibroblast Growth Factors
Interleukin-6
Platelet-Derived Growth Factor
Vascular Endothelial Growth Factor A
Figure
Reference
-
References
1. Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005; 23:1011–27.
Article2. Reddy S, Raffin M, Kaklamani V. Targeting angiogenesis in metastatic breast cancer. Oncologist. 2012; 17:1014–26.
Article3. Laird AD, Vajkoczy P, Shawver LK, Thurnher A, Liang C, Mohammadi M, et al. SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res. 2000; 60:4152–60.4. Suzuki Y, Saeki T, Aogi K, Toi M, Fujii H, Inoue K, et al. A multicenter phase II study of TSU-68, a novel oral multiple tyrosine kinase inhibitor, in patients with metastatic breast cancer progressing despite prior treatment with an anthracycline-containing regimen and taxane. Int J Clin Oncol. 2013; 18:590–7.
Article5. Toi M, Saeki T, Iwata H, Inoue K, Tokuda Y, Sato Y, et al. A multicenter phase II study of TSU-68, an oral multiple tyrosine kinase inhibitor, in combination with docetaxel in metastatic breast cancer patients with anthracycline resistance. Breast Cancer. 2014; 21:20–7.
Article6. Kim SB, Yoo C, Ro J, Im SA, Im YH, Kim JH, et al. Combination of docetaxel and TSU-68, an oral antiangiogenic agent, in patients with metastatic breast cancer previously treated with anthracycline: randomized phase II multicenter trial. Invest New Drugs. 2014; 32:753–61.
Article7. Murukesh N, Dive C, Jayson GC. Biomarkers of angiogenesis and their role in the development of VEGF inhibitors. Br J Cancer. 2010; 102:8–18.
Article8. Shin SJ, Jung M, Jeung HC, Kim HR, Rha SY, Roh JK, et al. A phase I pharmacokinetic study of TSU-68 (a multiple tyrosine kinase inhibitor of VEGFR-2, FGF and PDFG) in combination with S-1 and oxaliplatin in metastatic colorectal cancer patients previously treated with chemotherapy. Invest New Drugs. 2012; 30:1501–10.
Article9. Inaba Y, Kanai F, Aramaki T, Yamamoto T, Tanaka T, Yamakado K, et al. A randomised phase II study of TSU-68 in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. Eur J Cancer. 2013; 49:2832–40.
Article10. Pietras K, Sjoblom T, Rubin K, Heldin CH, Ostman A. PDGF receptors as cancer drug targets. Cancer Cell. 2003; 3:439–43.
Article11. Koizumi W, Yamaguchi K, Hosaka H, Takinishi Y, Nakayama N, Hara T, et al. Randomised phase II study of S-1/cisplatin plus TSU-68 vs S-1/cisplatin in patients with advanced gastric cancer. Br J Cancer. 2013; 109:2079–86.
Article12. Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci U S A. 2007; 104:17069–74.
Article13. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140:883–99.
Article14. Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009; 27:3437–44.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Improving Conventional or Low Dose Metronomic Chemotherapy with Targeted Antiangiogenic Drugs
- Application of Biomarkers for the Prediction and Diagnosis of Bone Metastasis in Breast Cancer
- Circulating Tumor Cells: Detection Methods and Potential Clinical Application in Breast Cancer
- Detection of Methylated Circulating DNA as Noninvasive Biomarkers for Breast Cancer Diagnosis
- Detection of Circulating Tumor Cells in Breast Cancer Patients Using Cytokeratin-19 Real-Time RT-PCR