Investig Magn Reson Imaging.
2019 Jun;23(2):81-99. 10.13104/imri.2019.23.2.81.
Deep Learning in MR Image Processing
- Affiliations
-
- 1Laboratory for Imaging Science and Technology, Department of Electrical and Computer Engineering, Institute of Engineering Research, Seoul National University, Seoul, Korea.
- 2Department of Electrical and Electronic Engineering, Yonsei University, Seoul, Korea.
- 3Department of Radiology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. yhnam83@gmail.com
- KMID: 2452523
- DOI: http://doi.org/10.13104/imri.2019.23.2.81
Abstract
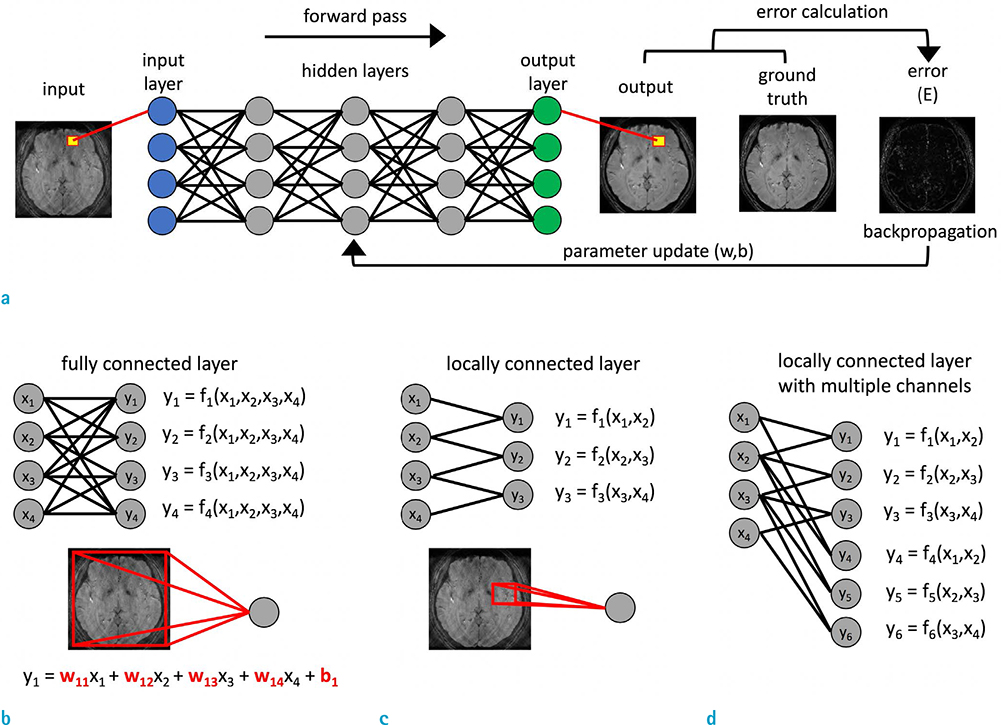
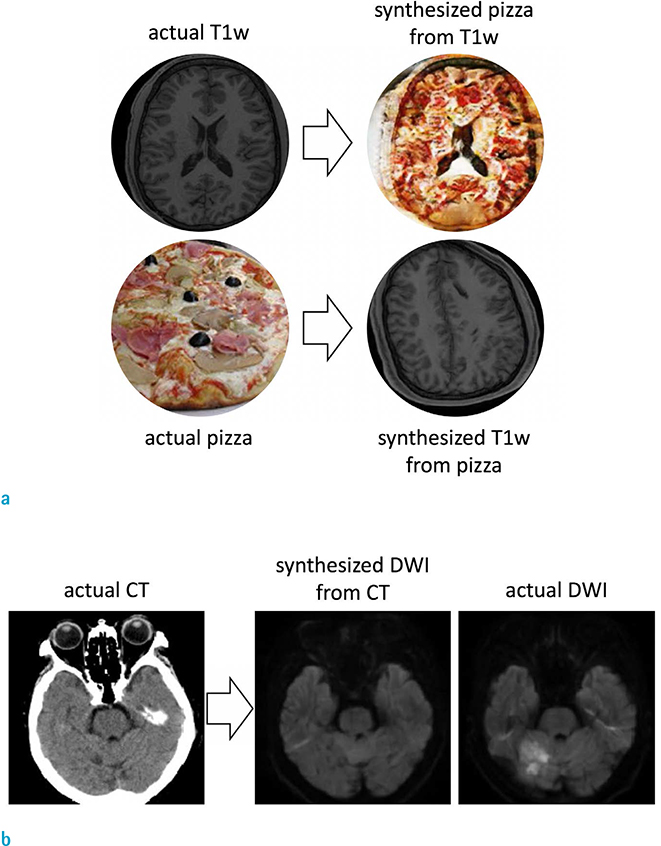
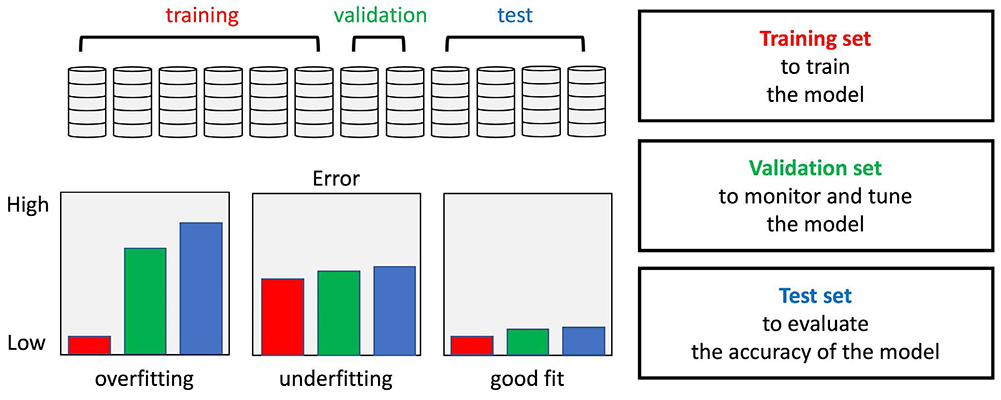
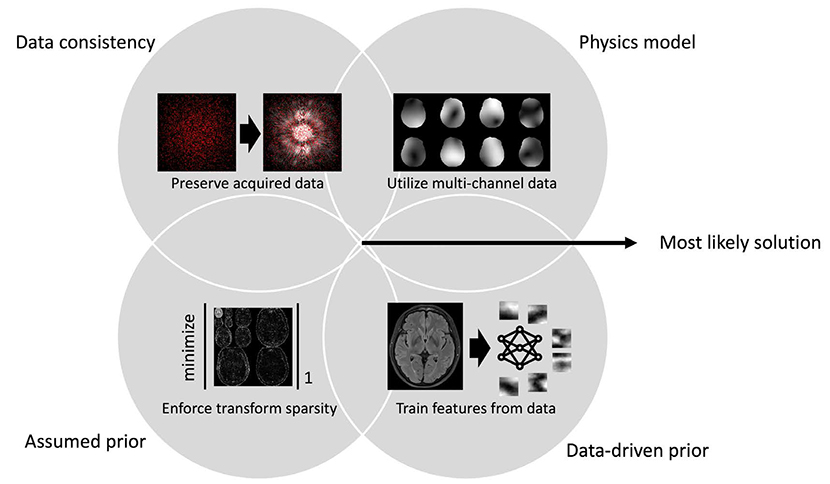
- Recently, deep learning methods have shown great potential in various tasks that involve handling large amounts of digital data. In the field of MR imaging research, deep learning methods are also rapidly being applied in a wide range of areas to complement or replace traditional model-based methods. Deep learning methods have shown remarkable improvements in several MR image processing areas such as image reconstruction, image quality improvement, parameter mapping, image contrast conversion, and image segmentation. With the current rapid development of deep learning technologies, the importance of the role of deep learning in MR imaging research appears to be growing. In this article, we introduce the basic concepts of deep learning and review recent studies on various MR image processing applications.
Keyword
MeSH Terms
Figure
Reference
-
1. Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. In : NIPS 2012: Neural Information Processing Systems; Lake Tahoe, Nevada. 2012. p. 1097–1105.2. Hinton G, Deng I, Yu D, et al. Deep neural networks for acoustic modeling in speech recognition: the shared views of four research groups. IEEE Signal Processing Magazine. 2012; 29:82–97.
Article3. Sutskever I, Vinyals O, Le QV. Sequence to sequence learning with neural networks. In : Proceedings of the 27th International Conference on Neural Information Processing Systems - Vol 2; 2014. p. 3104–3112.4. Jifara W, Jiang F, Rho S, Cheng M, Liu S. Medical image denoising using convolutional neural network: a residual learning approach. J Supercomput. 2017; 1–15.
Article5. Vincent P, Larochelle H, Lajoie I, Bengio Y, Manzagol PA. Stacked denoising autoencoders: Learning useful representations in a deep network with a local denoising criterion. J Mach Learn Res. 2010; 11:3371–3408.6. Zhang K, Zuo W, Chen Y, Meng D, Zhang L. Beyond a Gaussian denoiser: residual learning of deep CNN for image denoising. IEEE Trans Image Process. 2017; 26:3142–3155.
Article7. He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. In : Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR); 2016. p. 770–778.8. Simonyan K, Zisserman A. Very deep convolutional networks for large-scale image recognition. arXiv preprint arXiv:1409.1556. 2014.9. Chen LC, Papandreou G, Kokkinos I, Murphy K, Yuille AL. DeepLab: Semantic image segmentation with deep convolutional nets, atrous convolution, and fully connected CRFs. IEEE Trans Pattern Anal Mach Intell. 2018; 40:834–848.
Article10. Noh H, Hong S, Han B. Learning deconvolution network for semantic segmentation. In : Proceedings of the IEEE International Conference on Computer Vision (ICCV); 2015. p. 1520–1528.11. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015; 521:436–444.
Article12. Glorot X, Bordes A, Bengio Y. Deep sparse rectifier neural networks. In : Proceedings of the 14th International Conference on Artificial Intelligence and Statistics (AISTATS), 2011. Volume 15 of JMLR: W&CP 15; 2011. p. 315–323.13. LeCun Y, Boser B, Denker JS, et al. Handwritten digit recognition with a back-propagation network. Advances in neural information processing system 2. 1990. p. 396–404.14. LeCun Y, Bottou L, Bengio Y, Haffner P. Gradient-based learning applied to document recognition. Proceedings of the IEEE. 1998; 86:2278–2324.
Article15. Goodfellow I, Bengio Y, Courville A, Bengio Y. Deep learning. Cambridge: MIT Press;2016.16. Schmidhuber J. Deep learning in neural networks: an overview. Neural Netw. 2015; 61:85–117.
Article17. Szegedy C, Liu W, Jia Y, et al. Going deeper with convolutions. In : Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR); 2015. p. 1–9.18. Goodfellow I, Pouget-Abadie J, Mirza M, et al. Generative adversarial nets. In : Proceedings of the 27th International Conference on Neural Information Processing Systems; 2014. 2:p. 2672–2680.19. Isola P, Zhu J-Y, Zhou T, Efros AA. Image-to-image translation with conditional adversarial networks. In : Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR); 2017. p. 1125–1134.20. Zhu J-Y, Park T, Isola P, Efros AA. Unpaired image-toimage translation using cycle-consistent adversarial networks. In : Proceedings of the IEEE International Conference on Computer Vision (ICCV); 2017. p. 2223–2232.21. Zhu B, Liu JZ, Cauley SF, Rosen BR, Rosen MS. Image reconstruction by domain-transform manifold learning. Nature. 2018; 555:487–492.
Article22. Lauterbur PC. Image formation by induced local interactions. Examples employing nuclear magnetic resonance. 1973. Clin Orthop Relat Res. 1989; 3–6.23. Mansfield P, Maudsley AA. Medical imaging by NMR. Br J Radiol. 1977; 50:188–194.
Article24. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002; 47:1202–1210.
Article25. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999; 42:952–962.
Article26. Sodickson DK, Manning WJ. Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magn Reson Med. 1997; 38:591–603.
Article27. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007; 58:1182–1195.
Article28. Wang G, Ye JC, Mueller K, Fessler JA. Image reconstruction is a new frontier of machine learning. IEEE Trans Med Imaging. 2018; 37:1289–1296.
Article29. Boyd S, Vandenberghe L. Convex optimization. New York: Cambridge University Press;2004. p. 127–214.30. Han Y, Yoo J, Kim HH, Shin HJ, Sung K, Ye JC. Deep learning with domain adaptation for accelerated projection-reconstruction MR. Magn Reson Med. 2018; 80:1189–1205.
Article31. Ye JC, Han Y, Cha E. Deep convolutional framelets: a general deep learning framework for inverse problems. SIAM J Imaging Sci. 2018; 11:991–1048.
Article32. Lee D, Yoo J, Ye JC. Deep residual learning for compressed sensing MRI. In : IEEE 14th International Symposium on Biomedical Imaging (ISBI); 2017. p. 15–18.33. Hammernik K, Klatzer T, Kobler E, et al. Learning a variational network for reconstruction of accelerated MRI data. Magn Reson Med. 2018; 79:3055–3071.
Article34. Knoll F, Hammernik K, Kobler E, Pock T, Recht MP, Sodickson DK. Assessment of the generalization of learned image reconstruction and the potential for transfer learning. Magn Reson Med. 2019; 81:116–128.
Article35. Akcakaya M, Moeller S, Weingartner S, Ugurbil K. Scan-specific robust artificial-neural-networks for k-space interpolation (RAKI) reconstruction: Database-free deep learning for fast imaging. Magn Reson Med. 2019; 81:439–453.36. Yang G, Yu S, Dong H, et al. DAGAN: Deep de-aliasing generative adversarial networks for fast compressed sensing MRI reconstruction. IEEE Trans Med Imaging. 2018; 37:1310–1321.
Article37. Quan TM, Nguyen-Duc T, Jeong WK. Compressed sensing MRI reconstruction using a generative adversarial network with a cyclic loss. IEEE Trans Med Imaging. 2018; 37:1488–1497.
Article38. Mardani M, Gong E, Cheng JY, et al. Deep generative adversarial neural networks for compressive sensing MRI. IEEE Trans Med Imaging. 2019; 38:167–179.
Article39. Hyun CM, Kim HP, Lee SM, Lee S, Seo JK. Deep learning for undersampled MRI reconstruction. Phys Med Biol. 2018; 63:135007.
Article40. Eo T, Jun Y, Kim T, Jang J, Lee HJ, Hwang D. KIKInet: cross-domain convolutional neural networks for reconstructing undersampled magnetic resonance images. Magn Reson Med. 2018; 80:2188–2201.41. Aggarwal HK, Mani MP, Jacob M. MoDL: Model-based deep learning architecture for inverse problems. IEEE Trans Med Imaging. 2019; 38:394–405.
Article42. Benou A, Veksler R, Friedman A, Riklin Raviv T. Ensemble of expert deep neural networks for spatio-temporal denoising of contrast-enhanced MRI sequences. Med Image Anal. 2017; 42:145–159.
Article43. Dabov K, Foi A, Katkovnik V, Egiazarian K. Image restoration by sparse 3D transform-domain collaborative filtering. In : Image Processing: Algorithms and Systems VI; International Society for Optics and Photonics;2008. p. 681207.44. Elad M, Aharon M. Image denoising via sparse and redundant representations over learned dictionaries. IEEE Trans Image Process. 2006; 15:3736–3745.
Article45. Zoran D, Weiss Y. From learning models of natural image patches to whole image restoration. In : IEEE International Conference on Computer Vision (ICCV); 2011. p. 479–486.46. Gu S, Zhang L, Zuo W, Feng X. Weighted nuclear norm minimization with application to image denoising. In : IEEE Conference on Computer Vision and Pattern Recognition (CVPR); 2014. p. 2862–2869.47. Jin KH, McCann MT, Froustey E, Unser M. Deep convolutional neural network for inverse problems in imaging. IEEE Trans Image Process. 2017; 26:4509–4522.
Article48. Lee D, Yoo J, Tak S, Ye J. Deep residual learning for accelerated mri using magnitude and phase networks. IEEE Trans Biomed Eng. 2018; 65:1985–1995.
Article49. Kyathanahally SP, Doring A, Kreis R. Deep learning approaches for detection and removal of ghosting artifacts in MR spectroscopy. Magn Reson Med. 2018; 80:851–863.
Article50. Atkinson D, Hill DL, Stoyle PN, Summers PE, Keevil SF. Automatic correction of motion artifacts in magnetic resonance images using an entropy focus criterion. IEEE Trans Med Imaging. 1997; 16:903–910.
Article51. Loktyushin A, Nickisch H, Pohmann R, Scholkopf B. Blind retrospective motion correction of MR images. Magn Reson Med. 2013; 70:1608–1618.
Article52. Ooi MB, Krueger S, Thomas WJ, Swaminathan SV, Brown TR. Prospective real-time correction for arbitrary head motion using active markers. Magn Reson Med. 2009; 62:943–954.
Article53. Maclaren J, Armstrong BS, Barrows RT, et al. Measurement and correction of microscopic head motion during magnetic resonance imaging of the brain. PLoS One. 2012; 7:e48088.
Article54. Haskell MW, Cauley SF, Wald LL. TArgeted Motion Estimation and Reduction (TAMER): Data Consistency Based Motion Mitigation for MRI Using a Reduced Model Joint Optimization. IEEE Trans Med Imaging. 2018; 37:1253–1265.
Article55. Kober T, Marques JP, Gruetter R, Krueger G. Head motion detection using FID navigators. Magn Reson Med. 2011; 66:135–143.
Article56. Sommer K, Brosch T, Rafael W, et al. Correction of motion artifacts using a multi-resolution fully convolutional neural network. In : Proceeding ISMRM Scientific Meeting & Exhibition; 2018. p. 1175.57. Johnson PM, Drangova M. Motion correction in MRI using deep learning. In : Proceeding ISMRM Scientific Meeting & Exhibition; 2018. p. 4098.58. Pawar K, Chen Z, Shah J, Egan GF. Motion correction in MRI using deep convolutional neural network. In : Proceeding ISMRM Scientific Meeting & Exhibition; 2018. p. 1174.59. Lee H, Ryu K, Nam Y, Lee J, Kim DH. Reduction of respiratory motion artifact in c-spine imaging using deep learning: Is substitution of navigator possible? In : Proceeding ISMRM Scientific Meeting & Exhibition; 2018. p. 2660.60. Tamada D, Kromrey M-L, Onishi H, Motosugi U. Method for motion artifact reduction using a convolutional neural network for dynamic contrast enhanced MRI of the liver. arXiv preprint arXiv 2018:1807.06956.61. Meding K, Loktyushin A, Hirsch M. Automatic detection of motion artifacts in MR images using CNNS. In : 42nd IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP); 2017. p. 811–815.62. Pham CH, Ducournau A, Fablet R, Rousseau F. Brain MRI super-resolution using deep 3D convolutional networks. In : IEEE 14th International Symposium on Biomedical Imaging (ISBI); 2017. p. 197–200.63. Shi J, Liu Q, Wang C, Zhang Q, Ying S, Xu H. Superresolution reconstruction of MR image with a novel residual learning network algorithm. Phys Med Biol. 2018; 63:085011.
Article64. Chen Y, Xie Y, Zhou Z, Shi F, Christodoulou AG, Li D. Brain MRI super resolution using 3D deep densely connected neural networks. In : 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018); 2018. p. 739–742.65. Kim KH, Do WJ, Park SH. Improving resolution of MR images with an adversarial network incorporating images with different contrast. Med Phys. 2018; 45:3120–3131.66. Chaudhari AS, Fang Z, Kogan F, et al. Super-resolution musculoskeletal MRI using deep learning. Magn Reson Med. 2018; 80:2139–2154.67. Dong C, Loy CC, He K, Tang X. Image Super-Resolution Using Deep Convolutional Networks. IEEE Trans Pattern Anal Mach Intell. 2016; 38:295–307.
Article68. Tanenbaum LN, Tsiouris AJ, Johnson AN, et al. Synthetic MRI for Clinical Neuroimaging: Results of the Magnetic Resonance Image Compilation (MAGiC) Prospective, Multicenter, Multireader Trial. AJNR Am J Neuroradiol. 2017; 38:1103–1110.
Article69. Ryu K, Nam Y, Gho SM, et al. Data-driven synthetic MRI FLAIR artifact correction via deep neural network. J Magn Reson Imaging. 2019; [Epub ahead of print].
Article70. Lee J, Han Y, Ye JC. k-Space Deep Learning for Reference-free EPI Ghost Correction. arXiv preprint arXiv 2018:1806.00153v2.71. Kim KH, Choi SH, Park SH. Improving Arterial Spin Labeling by Using Deep Learning. Radiology. 2018; 287:658–666.
Article72. Golkov V, Dosovitskiy A, Sperl JI, et al. q-Space Deep Learning: Twelve-Fold Shorter and Model-Free Diffusion MRI Scans. IEEE Trans Med Imaging. 2016; 35:1344–1351.
Article73. Bertleff M, Domsch S, Weingartner S, et al. Diffusion parameter mapping with the combined intravoxel incoherent motion and kurtosis model using artificial neural networks at 3 T. NMR Biomed. 2017; 30:[Epub ahead of print].74. Domsch S, Murle B, Weingartner S, Zapp J, Wenz F, Schad LR. Oxygen extraction fraction mapping at 3 Tesla using an artificial neural network: a feasibility study. Magn Reson Med. 2018; 79:890–899.
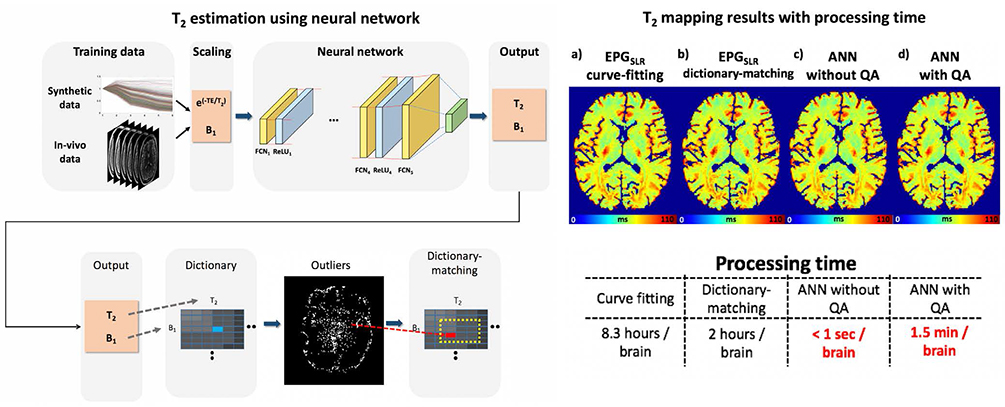
Article75. Lee D, Jung W, Lee J, et al. SafeNet: Artificial neural network for real-time T2 mapping with quality assurance. In : Joint Annual Meeting ISMRM-ESMRMB; ISMRM;2018. p. 2277.76. Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature. 2013; 495:187–192.
Article77. Cohen O, Zhu B, Rosen MS. MR fingerprinting Deep RecOnstruction NEtwork (DRONE). Magn Reson Med. 2018; 80:885–894.
Article78. Yoon J, Gong E, Chatnuntawech I, et al. Quantitative susceptibility mapping using deep neural network: QSMnet. Neuroimage. 2018; 179:199–206.
Article79. Shmueli K, de Zwart JA, van Gelderen P, Li TQ, Dodd SJ, Duyn JH. Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data. Magn Reson Med. 2009; 62:1510–1522.
Article80. Liu T, Spincemaille P, de Rochefort L, Kressler B, Wang Y. Calculation of susceptibility through multiple orientation sampling (COSMOS): a method for conditioning the inverse problem from measured magnetic field map to susceptibility source image in MRI. Magn Reson Med. 2009; 61:196–204.
Article81. Liu T, Liu J, de Rochefort L, et al. Morphology enabled dipole inversion (MEDI) from a single-angle acquisition: comparison with COSMOS in human brain imaging. Magn Reson Med. 2011; 66:777–783.
Article82. Wharton S, Schafer A, Bowtell R. Susceptibility mapping in the human brain using threshold-based k-space division. Magn Reson Med. 2010; 63:1292–1304.
Article83. de Rochefort L, Liu T, Kressler B, et al. Quantitative susceptibility map reconstruction from MR phase data using bayesian regularization: validation and application to brain imaging. Magn Reson Med. 2010; 63:194–206.
Article84. Papyan V, Romano Y, Elad M. Convolutional Neural Networks Analyzed via Convolutional Sparse Coding. J Mach Learn Res. 2017; 83:1–52.85. Wiatowski T, Bolcskei H. A Mathematical Theory of Deep Convolutional Neural Networks for Feature Extraction. IEEE Trans Inf Theory. 2018; 64:1845–1866.
Article86. Ye JC, Han Y, Cha E. Deep Convolutional Framelets: A General Deep Learning Framework for Inverse Problems. SIAM J Imaging Sci. 2018; 11:991–1048.
Article87. Razavian AS, Azizpour H, Sullivan J, Carlsson S. CNN features off-the-shelf: an astounding baseline for recognition. arXiv preprint arXiv 2014:1403.6382.88. Yosinski J, Clune J, Bengio Y, Lipson H. How transferable are features in deep neural networks?. In : Ghahramani Z, Welling M, Cortes C, Lawrence ND, Weinberger KQ, editors. Advances in neural information processing systems 27. Curran Associates, Inc.;2014. p. 3320–3328.89. Knoll F, Hammernik K, Kobler E, Pock T, Recht MP, Sodickson DK. Assessment of the generalization of learned image reconstruction and the potential for transfer learning. Magn Reson Med. 2019; 81:116–128.
Article90. Fong RC, Vedaldi A. Interpretable explanations of black boxes by meaningful perturbation. In : IEEE International Conference on Computer Vision (ICCV); 2017. p. 3449–3457.91. Zeiler MD, Fergus R. Visualizing and understanding convolutional networks. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). Volume 8689 LNCS, No. PART 1. Springer Verlag;2014. p. 818–833.92. Liu F, Jang H, Kijowski R, Bradshaw T, McMillan AB. Deep learning MR imaging-based attenuation correction for PET/MR imaging. Radiology. 2018; 286:676–684.
Article93. Han X. MR-based synthetic CT generation using a deep convolutional neural network method. Med Phys. 2017; 44:1408–1419.
Article94. Xiang L, Wang Q, Nie D, et al. Deep embedding convolutional neural network for synthesizing CT image from T1-Weighted MR image. Med Image Anal. 2018; 47:31–44.
Article95. Wolterink JM, Dinkla AM, Savenije MHF, Seevinck PR, van den Berg CAT, Isgum I. Deep MR to CT synthesis using unpaired data. In : International Workshop on Simulation and Synthesis in Medical Imaging; Cham: Springer;2017. p. 14–23.96. Jun Y, Eo T, Kim T, et al. Deep-learned 3D black-blood imaging using automatic labelling technique and 3D convolutional neural networks for detecting metastatic brain tumors. Sci Rep. 2018; 8:9450.
Article97. Gong E, Pauly JM, Wintermark M, Zaharchuk G. Deep learning enables reduced gadolinium dose for contrast-enhanced brain MRI. J Magn Reson Imaging. 2018; 48:330–340.
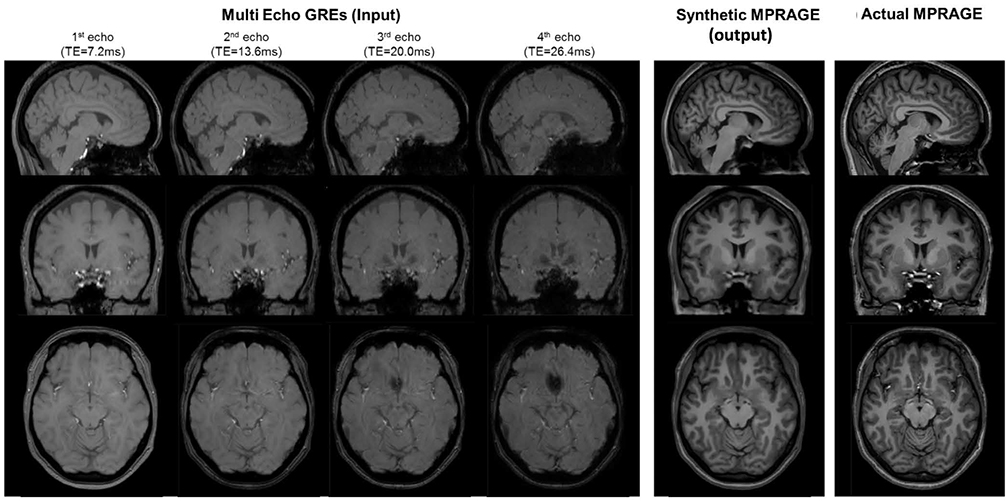
Article98. Ryu K, Shin NY, Kim DH, Nam Y. Synthesizing T1 weighted MPRAGE image from multi echo GRE images via deep neural network. Magn Reson Imaging. 2019; [Epub ahead of print].
Article99. Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. In : International Conference on Medical Image Computing and Computer-assisted Invervention; 2015. p. 234–241.100. Long J, Shelhamer E, Darrell T. Fully convolutional networks for semantic segmentation. In : Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR); Cham: Springer;2015. p. 3431–3440.101. Yang Q, Yan P, Zhang Y, et al. Low-Dose CT Image Denoising Using a Generative Adversarial Network With Wasserstein Distance and Perceptual Loss. IEEE Trans Med Imaging. 2018; 37:1348–1357.
Article102. Nie D, Trullo R, Lian J, et al. Medical image synthesis with context-aware generative adversarial networks. In : Medical Image Computing and Computer-Assisted Intervention-MICCAI 2017-20th International Conference, Proceedings; Springer Verlag;2017. p. 417–425.103. Fischl B. FreeSurfer. Neuroimage. 2012; 62:774–781.
Article104. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012; 62:782–790.
Article105. Kamnitsas K, Chen L, Ledig C, Rueckert D, Glocker B. Multi-scale 3D convolutional neural networks for lesion segmentation in brain MRI. Ischemic Stroke Lesion Segment. 2015; 13:46.106. Rajchl M, Pawlowski N, Rueckert D, Matthews PM, Glocker B. NeuroNet: Fast and robust reproduction of multiple brain image segmentation pipelines. arXiv preprint arXiv 2018:1806.04224.107. Havaei M, Davy A, Warde-Farley D, et al. Brain tumor segmentation with deep neural networks. Med Image Anal. 2017; 35:18–31.
Article108. Pereira S, Pinto A, Alves V, Silva CA. Brain tumor segmentation using convolutional neural networks in MRI images. IEEE Trans Med Imaging. 2016; 35:1240–1251.
Article109. Akkus Z, Galimzianova A, Hoogi A, Rubin DL, Erickson BJ. Deep learning for brain MRI segmentation: state of the art and future directions. J Digit Imaging. 2017; 30:449–459.
Article110. Liu S, Zheng H, Feng Y, Li W. Prostate cancer diagnosis using deep learning with 3D multiparametric MRI. SPIE Med Imaging. 2017; 10134:1–4.
Article111. Song Y, Zhang YD, Yan X, et al. Computer-aided diagnosis of prostate cancer using a deep convolutional neural network from multiparametric MRI. J Magn Reson Imaging. 2018; 48:1570–1577.
Article112. Milletari F, Navab N, Ahmadi S-A. V-net: fully convolutional neural networks for volumetric medical image segmentation. In : 2016 Fourth International Conference on 3D Vision (3DV); IEEE;2016. p. 565–571.113. Guerrero R, Qin C, Oktay O, et al. White matter hyperintensity and stroke lesion segmentation and differentiation using convolutional neural networks. Neuroimage Clin. 2018; 17:918–934.
Article114. Norman B, Pedoia V, Majumdar S. Use of 2D U-net convolutional neural networks for automated cartilage and meniscus segmentation of knee MR imaging data to determine relaxometry and morphometry. Radiology. 2018; 288:177–185.115. Prasoon A, Petersen K, Igel C, Lauze F, Dam E, Nielsen M. Deep feature learning for knee cartilage segmentation using a triplanar convolutional neural network. Med image Comput Comput Assist Interv. 2013; 16:246–253.
Article116. Zhou Z, Zhao G, Kijowski R, Liu F. Deep convolutional neural network for segmentation of knee joint anatomy. Magn Reson Med. 2018; 80:2759–2770.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deep Learning in MR Motion Correction:a Brief Review and a New Motion Simulation Tool (view2Dmotion)
- Deep Learning Applications in Perfusion MRI: Recent Advances and Current Challenges
- Deep Learning in Dental Radiographic Imaging
- Data Augmentation Techniques for Deep Learning-Based Medical Image Analyses
- Applications of Artificial Intelligence in MR Image Acquisition and Reconstruction