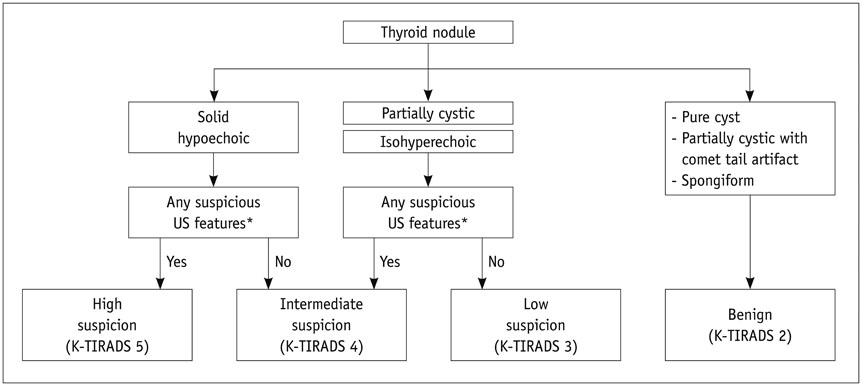
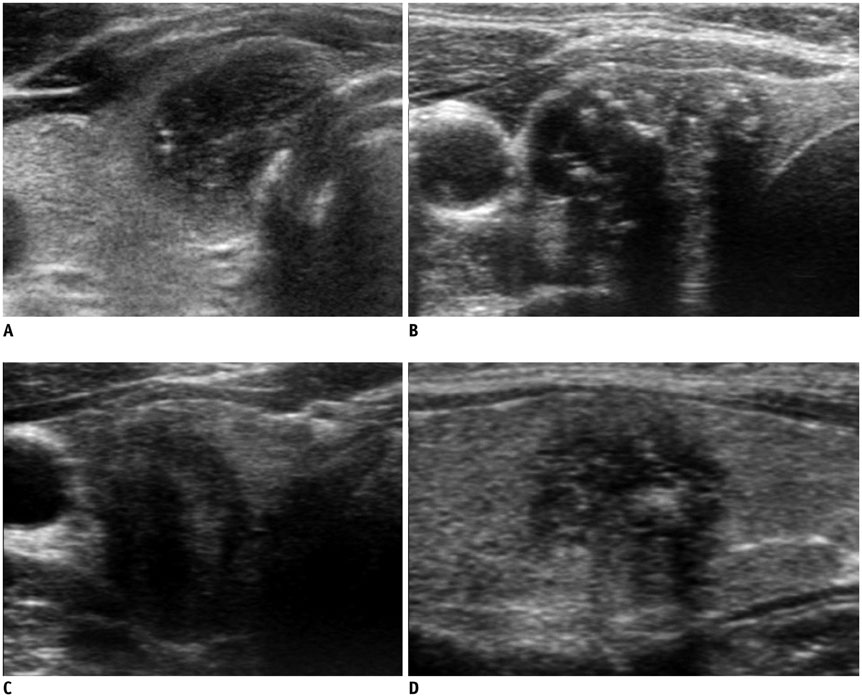
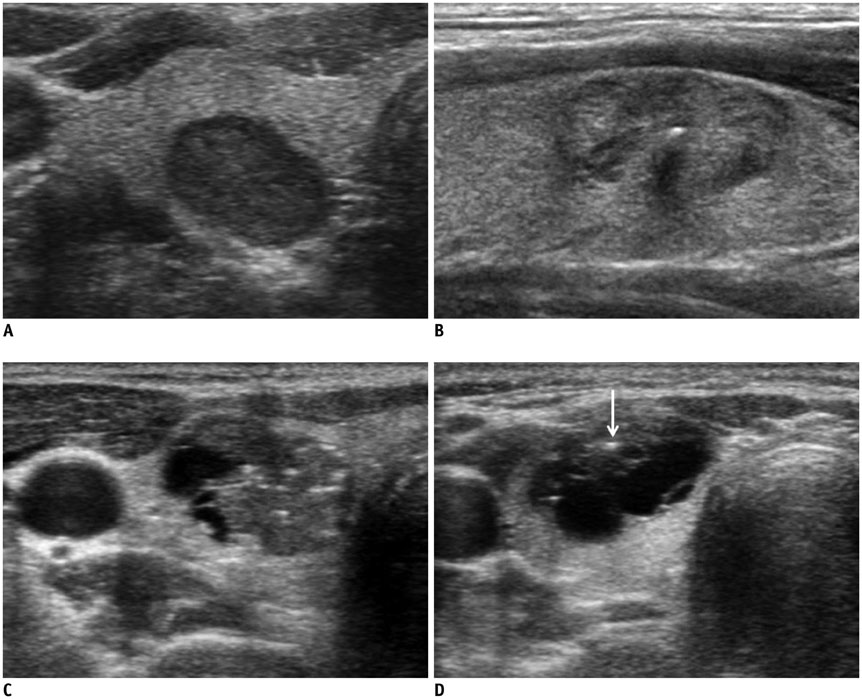
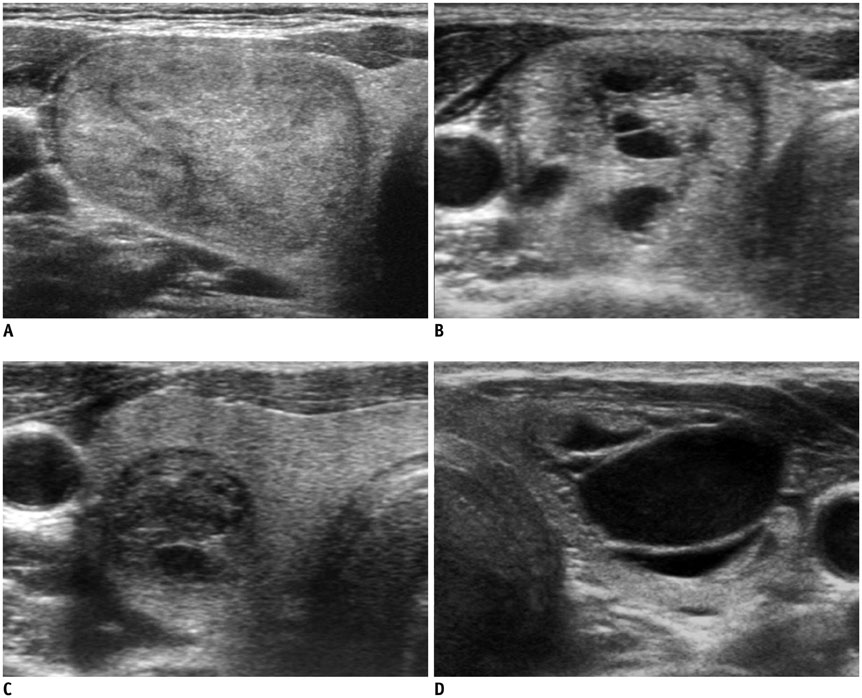
Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 06351, Korea.
- 2Department of Radiology and Research Institute of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul 05505, Korea.
- 3Department of Radiology, Ewha Womans University School of Medicine, Seoul 07985, Korea.
- 4Department of Radiology, Ajou University School of Medicine, Suwon 16499, Korea.
- 5Department of Radiology, Seoul National University College of Medicine, Seoul 03080, Korea.
- 6Department of Radiology, Ansan Hospital, Korea University College of Medicine, Ansan 15355, Korea.
- 7Department of Radiology, Soonchunhyang University Seoul Hospital, Seoul 04401, Korea.
- 8Department of Radiology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul 05030, Korea.
- 9Department of Radiology, Human Medical Imaging and Intervention Center, Seoul 06524, Korea. nndgna@gmail.com
- 10Department of Radiology, Hanyang University College of Medicine, Hanyang University Hospital, Seoul 04763, Korea.
- 11Department of Radiology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul 03181, Korea.
- 12Department of Radiology, Wonkwang University Hospital, Iksan 54538, Korea.
- 13Department of Radiology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- 14Department of Radiology, Busan Paik Hospital, Inje University College of Medicine, Busan 47392, Korea.
- 15Department of Radiology, Severance Hospital, Research Institute of Radiological Science, Yonsei University College of Medicine, Seoul 03722, Korea.
- 16Department of Radiology, Research Institute and Hospital, National Cancer Center, Goyang 10408, Korea.
- 17Department of Radiology, Kyungpook National University Hospital, Daegu 41944, Korea.
- 18Department of Radiology, Dong-A University Medical Center, Busan 49201, Korea.
- 19Department of Radiology, Newwoori Namsan Hospital, Busan 46224, Korea.
- 20Department of Radiology, SMG-SNU Boramae Medical Center, Seoul National University College of Medicine, Seoul 07061, Korea.
- 21Department of Radiology and Thyroid Center, Daerim St. Mary's Hospital, Seoul 07442, Korea.
- KMID: 2451412
- DOI: http://doi.org/10.3348/kjr.2016.17.3.370
Abstract
- The rate of detection of thyroid nodules and carcinomas has increased with the widespread use of ultrasonography (US), which is the mainstay for the detection and risk stratification of thyroid nodules as well as for providing guidance for their biopsy and nonsurgical treatment. The Korean Society of Thyroid Radiology (KSThR) published their first recommendations for the US-based diagnosis and management of thyroid nodules in 2011. These recommendations have been used as the standard guidelines for the past several years in Korea. Lately, the application of US has been further emphasized for the personalized management of patients with thyroid nodules. The Task Force on Thyroid Nodules of the KSThR has revised the recommendations for the ultrasound diagnosis and imaging-based management of thyroid nodules. The review and recommendations in this report have been based on a comprehensive analysis of the current literature and the consensus of experts.
Keyword
MeSH Terms
-
Elasticity Imaging Techniques
Endoscopic Ultrasound-Guided Fine Needle Aspiration
Humans
Image Processing, Computer-Assisted
Lymph Nodes/diagnostic imaging
Multidetector Computed Tomography
Practice Guidelines as Topic
Societies, Medical
Thyroid Neoplasms/*diagnosis/diagnostic imaging/pathology
Thyroid Nodule/*diagnosis/diagnostic imaging/pathology
Ultrasonography
Figure
Cited by 39 articles
-
Thyroid nodules with discordant results of ultrasonographic and fine-needle aspiration findings
Min Joo Kim, Ka Hee Yi
J Korean Med Assoc. 2018;61(4):225-231. doi: 10.5124/jkma.2018.61.4.225.Ultrasonographic Characteristics of the Hyperfunctioning Thyroid Nodule and Predictive Factors for Thyroid Stimulating Hormone Suppression
Won Sang Yoo, Hoon Sung Choi
Int J Thyroidol. 2019;12(1):35-43. doi: 10.11106/ijt.2019.12.1.35.The Incidence and Clinicopathologic Characteristics of Patients Who Had False-Positive Fine-Needle Aspiration Results for Papillary Thyroid Cancer
Yoonju Bang, Kyorim Back, Jung-Han Kim, Junho Choe, Jee Soo Kim
J Endocr Surg. 2019;19(4):136-143. doi: 10.16956/jes.2019.19.4.136.Clinical Outcome of Fine Needle Aspiration Cytology and Washout Thyroglobulin in Suspicious Lymph Nodes in Differentiated Thyroid Carcinoma: Discordant Results in Real-World Practice
Jeongmin Lee, Hye Lim Park, Kwanhoon Jo, Min-Hee Kim, Ja Seong Bae, Sohee Lee, Chan Kwon Jung, So-Lyung Jung, Dong-Jun Lim
Int J Thyroidol. 2021;14(1):18-27. doi: 10.11106/ijt.2021.14.1.18.Revisiting Rupture of Benign Thyroid Nodules after Radiofrequency Ablation: Various Types and Imaging Features
Sae Rom Chung, Jung Hwan Baek, Jin Yong Sung, Ji Hwa Ryu, So Lyung Jung
Endocrinol Metab. 2019;34(4):415-421. doi: 10.3803/EnM.2019.34.4.415.Innovative Techniques for Image-Guided Ablation of Benign Thyroid Nodules: Combined Ethanol and Radiofrequency Ablation
Hye Sun Park, Jung Hwan Baek, Young Jun Choi, Jeong Hyun Lee
Korean J Radiol. 2017;18(3):461-469. doi: 10.3348/kjr.2017.18.3.461.RE: Thyroid Core Needle Biopsy: The Strengths of Guidelines of the Korean Society of Thyroid Radiology
Anna Crescenzi, Pierpaolo Trimboli
Korean J Radiol. 2017;18(5):867-869. doi: 10.3348/kjr.2017.18.5.867.Columnar Cell Variant of Papillary Thyroid Carcinoma: Ultrasonographic and Clinical Differentiation between the Indolent and Aggressive Types
Jooyeon Cho, Jung Hee Shin, Soo Yeon Hahn, Young Lyun Oh
Korean J Radiol. 2018;19(5):1000-1005. doi: 10.3348/kjr.2018.19.5.1000.Ultrasonographic Interval Changes in Solid Thyroid Nodules after Ultrasonography-Guided Fine-Needle Aspiration
Ik Jung Hwang, Dong Wook Kim, Yoo Jin Lee, Hye Jung Choo, Soo Jin Jung, Hye Jin Baek
Korean J Radiol. 2018;19(1):158-166. doi: 10.3348/kjr.2018.19.1.158.Quality of Life in Patients Treated with Percutaneous Laser Ablation for Non-Functioning Benign Thyroid Nodules: A Prospective Single-Center Study
Silvia Oddo, Edineia Felix, Michele Mussap, Massimo Giusti
Korean J Radiol. 2018;19(1):175-184. doi: 10.3348/kjr.2018.19.1.175.Impact of Nodule Size on Malignancy Risk Differs according to the Ultrasonography Pattern of Thyroid Nodules
Min Ji Hong, Dong Gyu Na, Jung Hwan Baek, Jin Yong Sung, Ji-Hoon Kim
Korean J Radiol. 2018;19(3):534-541. doi: 10.3348/kjr.2018.19.3.534.Primary Imaging Test and Appropriate Biopsy Methods for Thyroid Nodules: Guidelines by Korean Society of Radiology and National Evidence-Based Healthcare Collaborating Agency
Eun Ju Ha, Hyun Kyung Lim, Jung Hyun Yoon, Jung Hwan Baek, Kyung Hyun Do, Miyoung Choi, Jin A Choi, Min Lee, Dong Gyu Na,
Korean J Radiol. 2018;19(4):623-631. doi: 10.3348/kjr.2018.19.4.623.Computer-Aided Diagnosis of Thyroid Nodules via Ultrasonography: Initial Clinical Experience
Young Jin Yoo, Eun Ju Ha, Yoon Joo Cho, Hye Lin Kim, Miran Han, So Young Kang
Korean J Radiol. 2018;19(4):665-672. doi: 10.3348/kjr.2018.19.4.665.Evaluation of Modified Core-Needle Biopsy in the Diagnosis of Thyroid Nodules
Soomin Ahn, Sejin Jung, Ji-Ye Kim, Jung Hee Shin, Soo Yeon Hahn, Young Lyun Oh
Korean J Radiol. 2018;19(4):656-664. doi: 10.3348/kjr.2018.19.4.656.2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology
Ji-hoon Kim, Jung Hwan Baek, Hyun Kyung Lim, Hye Shin Ahn, Seon Mi Baek, Yoon Jung Choi, Young Jun Choi, Sae Rom Chung, Eun Ju Ha, Soo Yeon Hahn, So Lyung Jung, Dae Sik Kim, Soo Jin Kim, Yeo Koon Kim, Chang Yoon Lee, Jeong Hyun Lee, Kwang Hwi Lee, Young Hen Lee, Jeong Seon Park, Hyesun Park, Jung Hee Shin, Chong Hyun Suh, Jin Yong Sung, Jung Suk Sim, Inyoung Youn, Miyoung Choi, Dong Gyu Na,
Korean J Radiol. 2018;19(4):632-655. doi: 10.3348/kjr.2018.19.4.632.Complementary Role of Elastography Using Carotid Artery Pulsation in the Ultrasonographic Assessment of Thyroid Nodules: A Prospective Study
Soo Yeon Hahn, Jung Hee Shin, Eun Young Ko, Jung Min Bae, Ji Soo Choi, Ko Woon Park
Korean J Radiol. 2018;19(5):992-999. doi: 10.3348/kjr.2018.19.5.992.Ultrasound-Guided Core Needle Biopsy Techniques for Intermediate or Low Suspicion Thyroid Nodules: Which Method is Effective for Diagnosis?
Soo Yeon Hahn, Jung Hee Shin, Young Lyun Oh, Ko Woon Park
Korean J Radiol. 2019;20(10):1454-1461. doi: 10.3348/kjr.2018.0841.The Role of Core Needle Biopsy for the Evaluation of Thyroid Nodules with Suspicious Ultrasound Features
Sae Rom Chung, Jung Hwan Baek, Young Jun Choi, Tae-Yon Sung, Dong Eun Song, Tae Yong Kim, Jeong Hyun Lee
Korean J Radiol. 2019;20(1):158-165. doi: 10.3348/kjr.2018.0101.US-Guided Radiofrequency Ablation for Low-Risk Papillary Thyroid Microcarcinoma: Efficacy and Safety in a Large Population
Hyun Kyung Lim, Se Jin Cho, Jung Hwan Baek, Kang Dae Lee, Chang Woo Son, Jung Min Son, Seon Mi Baek
Korean J Radiol. 2019;20(12):1653-1661. doi: 10.3348/kjr.2019.0192.Degenerating Thyroid Nodules: Ultrasound Diagnosis, Clinical Significance, and Management
Jie Ren, Jung Hwan Baek, Sae Rom Chung, Young Jun Choi, Chan Kwon Jung, Jeong Hyun Lee
Korean J Radiol. 2019;20(6):947-955. doi: 10.3348/kjr.2018.0599.Ethanol Ablation of the Thyroid Nodules: 2018 Consensus Statement by the Korean Society of Thyroid Radiology
Soo Yeon Hahn, Jung Hee Shin, Dong Gyu Na, Eun Joo Ha, Hye Shin Ahn, Hyun Kyung Lim, Jeong Hyun Lee, Jeong Seon Park, Ji-hoon Kim, Jin Yong Sung, Joon Hyung Lee, Jung Hwan Baek, Jung Hyun Yoon, Jung Suk Sim, Kwang Hwi Lee, Seon Mi Baek, So Lyung Jung, Yeo Koon Kim, Yoon Jung Choi, ,
Korean J Radiol. 2019;20(4):609-620. doi: 10.3348/kjr.2018.0696.History of Korean Society of Thyroid Radiology
Dong Gyu Na, Jung Hwan Baek
Int J Thyroidol. 2018;11(1):11-14. doi: 10.11106/ijt.2018.11.1.11.2016 Revised Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Thyroid Cancer
Ka Hee Yi, Eun Kyung Lee, Ho-Cheol Kang, Sun Wook Kim, In Joo Kim, So Yeon Park, Kee-Hyun Nam, Jin Woo Park, Sang Kyun Bae, Seung-Kuk Baek, Jung Hwan Baek, Byung-Joo Lee, Ki-Wook Chung, Yuh-Seog Jung, Gi Jeong Cheon, Won Bae Kim, Jae Hoon Chung, Young-Soo Rho, Dong Gyu Na, Yunwoo Koh
Int J Thyroidol. 2016;9(2):59-126. doi: 10.11106/ijt.2016.9.2.59.Predictive Factors for Occult Contralateral Papillary Thyroid Carcinoma in Patients with Ipsilateral Multifocality on Frozen Biopsy
Ki Nam Park, Se A Lee, Sang Kuk Lee, Jae Hyun Jeong, Sang Woo Sun, Jung Ja Gwak, Seung Won Lee
Korean J Otorhinolaryngol-Head Neck Surg. 2017;60(10):517-521. doi: 10.3342/kjorl-hns.2017.00248.Cervical Bronchogenic Cysts Mimicking Papillary Thyroid Carcinoma on Ultrasound
Dongbin Ahn, Jeong Kyu Kim
Korean J Otorhinolaryngol-Head Neck Surg. 2019;62(12):735-739. doi: 10.3342/kjorl-hns.2019.00080.Diagnostic Performance of a Combination of Shear Wave Elastography and B-Mode Ultrasonography in Differentiating Benign From Malignant Thyroid Nodules
Eung Koo Yeon, Yu-Mee Sohn, Mirinae Seo, Eui-Jong Kim, Young-Gyu Eun, Won Seo Park, Seong Jong Yun
Clin Exp Otorhinolaryngol. 2020;13(2):186-193. doi: 10.21053/ceo.2019.01235.Association of Hyperparathyroidism and Papillary Thyroid Cancer: A Multicenter Retrospective Study
Chaiho Jeong, Hye In Kwon, Hansang Baek, Hun-Sung Kim, Dong-Jun Lim, Ki-Hyun Baek, Jeonghoon Ha, Moo Il Kang
Endocrinol Metab. 2020;35(4):925-932. doi: 10.3803/EnM.2020.725.Association of Hyperparathyroidism and Papillary Thyroid Cancer: A Multicenter Retrospective Study (
Endocrinol Metab 2020;35:925–32, Chaiho Jeong et al.)
Chaiho Jeong, Jeonghoon Ha, Moo Il Kang
Endocrinol Metab. 2021;36(1):205-206. doi: 10.3803/EnM.2021.100.Protocol for a Korean Multicenter Prospective Cohort Study of Active Surveillance or Surgery (KoMPASS) in Papillary Thyroid Microcarcinoma
Min Ji Jeon, Yea Eun Kang, Jae Hoon Moon, Dong Jun Lim, Chang Yoon Lee, Yong Sang Lee, Sun Wook Kim, Min-Hee Kim, Bo Hyun Kim, Ho-Cheol Kang, Minho Shong, Sun Wook Cho, Won Bae Kim
Endocrinol Metab. 2021;36(2):359-364. doi: 10.3803/EnM.2020.890.A Multicenter, Randomized, Controlled Trial for Assessing the Usefulness of Suppressing Thyroid Stimulating Hormone Target Levels after Thyroid Lobectomy in Low to Intermediate Risk Thyroid Cancer Patients (MASTER): A Study Protocol
Eun Kyung Lee, Yea Eun Kang, Young Joo Park, Bon Seok Koo, Ki-Wook Chung, Eu Jeong Ku, Ho-Ryun Won, Won Sang Yoo, Eonju Jeon, Se Hyun Paek, Yong Sang Lee, Dong Mee Lim, Yong Joon Suh, Ha Kyoung Park, Hyo-Jeong Kim, Bo Hyun Kim, Mijin Kim, Sun Wook Kim, Ka Hee Yi, Sue K. Park, Eun-Jae Jung, June Young Choi, Ja Seong Bae, Joon Hwa Hong, Kee-Hyun Nam, Young Ki Lee, Hyeong Won Yu, Sujeong Go, Young Mi Kang
Endocrinol Metab. 2021;36(3):574-581. doi: 10.3803/EnM.2020.943.Clinicopathological Characteristics and Disease-Free Survival in Patients with Hürthle Cell Carcinoma: A Multicenter Cohort Study in South Korea
Meihua Jin, Eun Sook Kim, Bo Hyun Kim, Hee Kyung Kim, Yea Eun Kang, Min Ji Jeon, Tae Yong Kim, Ho-Cheol Kang, Won Bae Kim, Young Kee Shong, Mijin Kim, Won Gu Kim
Endocrinol Metab. 2021;36(5):1078-1085. doi: 10.3803/EnM.2021.1151.Comparison of Korean vs. American Thyroid Imaging Reporting and Data System in Malignancy Risk Assessment of Indeterminate Thyroid Nodules
Sunyoung Kang, Seul Ki Kwon, Hoon Sung Choi, Min Joo Kim, Young Joo Park, Do Joon Park, Sun Wook Cho
Endocrinol Metab. 2021;36(5):1111-1120. doi: 10.3803/EnM.2021.1208.Comparison of Thyroid Imaging Reporting and Data Systems in Malignancy Risk Stratification of Indeterminate Thyroid Nodules
Bo Hyun Kim
Endocrinol Metab. 2021;36(5):974-976. doi: 10.3803/EnM.2021.1287.Lower Thyroid Cancer Mortality in Patients Detected by Screening: A Meta-Analysis
Shinje Moon, Young Shin Song, Kyong Yeun Jung, Eun Kyung Lee, Young Joo Park
Endocrinol Metab. 2023;38(1):93-103. doi: 10.3803/EnM.2023.1667.Diagnostic Performance of Ultrasound-Based Risk Stratification Systems for Thyroid Nodules: A Systematic Review and Meta-Analysis
Leehi Joo, Min Kyoung Lee, Ji Ye Lee, Eun Ju Ha, Dong Gyu Na
Endocrinol Metab. 2023;38(1):117-128. doi: 10.3803/EnM.2023.1670.Guideline for the Surgical Management of Locally Invasive Differentiated Thyroid Cancer From the Korean Society of Head and Neck Surgery
Jun-Ook Park, Joo Hyun Kim, Young Hoon Joo, Sang-Yeon Kim, Geun-Jeon Kim, Hyun Bum Kim, Dong-Hyun Lee, Hyun Jun Hong, Young Min Park, Eun-Jae Chung, Yong Bae Ji, Kyoung Ho Oh, Hyoung Shin Lee, Dong Kun Lee, Ki Nam Park, Myung Jin Ban, Bo Hae Kim, Do Hun Kim, Jae-Keun Cho, Dong Bin Ahn, Min-Su Kim, Jun Girl Seok, Jeon Yeob Jang, Hyo Geun Choi, Hee Jin Kim, Sung Joon Park, Eun Kyung Jung, Yeon Soo Kim, Yong Tae Hong, Young Chan Lee, Ho-Ryun Won, Sung-Chan Shin, Seung-Kuk Baek, Soon Young Kwon
Clin Exp Otorhinolaryngol. 2023;16(1):1-19. doi: 10.21053/ceo.2022.01732.2023 Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules
Young Joo Park, Eun Kyung Lee, Young Shin Song, Soo Hwan Kang, Bon Seok Koo, Sun Wook Kim, Dong Gyu Na, Seung-Kuk Baek, So Won Oh, Min Kyoung Lee, Sang-Woo Lee, Young Ah Lee, Yong Sang Lee, Ji Ye Lee, Dong-Jun Lim, Leehi Joo, Yuh-Seog Jung, Chan Kwon Jung, Yoon Young Cho, Yun Jae Chung, Won Bae Kim, Ka Hee Yi, Ho-Cheol Kang, Do Joon Park
Int J Thyroidol. 2023;16(1):1-31. doi: 10.11106/ijt.2023.16.1.1.Strategies for Safe and Effective Core Needle Biopsy of Thyroid Nodules with Macrocalcification
Jae Ho Shin
Int J Thyroidol. 2023;16(2):195-199. doi: 10.11106/ijt.2023.16.2.195.Korean Thyroid Association Management Guidelines for Patients with Thyroid Nodules 2024
Young Joo Park, Eun Kyung Lee, Young Shin Song, Su Hwan Kang, Bon Seok Koo, Sun Wook Kim, Dong Gyu Na, Seung-Kuk Baek, So Won Oh, Min Kyoung Lee, Sang-Woo Lee, Young Ah Lee, Yong Sang Lee, Ji Ye Lee, Dong-Jun Lim, Leehi Joo, Yuh-Seog Jung, Chan Kwon Jung, Yoon Young Cho, Yun Jae Chung, Won Bae Kim, Ka Hee Yi, Ho-Cheol Kang, Do Joon Park
Int J Thyroidol. 2024;17(1):208-244. doi: 10.11106/ijt.2024.17.1.208.
Reference
-
1. Vander JB, Gaston EA, Dawber TR. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Ann Intern Med. 1968; 69:537–554.2. Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf). 1977; 7:481–449.3. Mandel SJ. A 64-year-old woman with a thyroid nodule. JAMA. 2004; 292:2632–2642.4. Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002; 87:1941–1946.5. Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf). 2004; 60:21–22.6. Smith-Bindman R, Lebda P, Feldstein VA, Sellami D, Goldstein RB, Brasic N, et al. Risk of thyroid cancer based on thyroid ultrasound imaging characteristics: results of a population-based study. JAMA Intern Med. 2013; 173:1788–1796.7. Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid. 2015; 25:1127–1136.8. Ahn HY, Park YJ. Incidence and clinical characteristics of thyroid cancer in Korea. Korean J Med. 2009; 77:537–542.9. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006; 295:2164–2167.10. Gharib H, Hegedüs L, Pacella CM, Baek JH, Papini E. Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab. 2013; 98:3949–3957.11. Moon WJ, Baek JH, Jung SL, Kim DW, Kim EK, Kim JY, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol. 2011; 12:1–14.12. Shin JJ, Caragacianu D, Randolph GW. Impact of thyroid nodule size on prevalence and post-test probability of malignancy: a systematic review. Laryngoscope. 2015; 125:263–272.13. Kamran SC, Marqusee E, Kim MI, Frates MC, Ritner J, Peters H, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013; 98:564–570.14. McHenry CR, Huh ES, Machekano RN. Is nodule size an independent predictor of thyroid malignancy? Surgery. 2008; 144:1062–1068.15. Shrestha M, Crothers BA, Burch HB. The impact of thyroid nodule size on the risk of malignancy and accuracy of fine-needle aspiration: a 10-year study from a single institution. Thyroid. 2012; 22:1251–1256.16. Asanuma K, Kobayashi S, Shingu K, Hama Y, Yokoyama S, Fujimori M, et al. The rate of tumour growth does not distinguish between malignant and benign thyroid nodules. Eur J Surg. 2001; 167:102–105.17. Alexander EK, Hurwitz S, Heering JP, Benson CB, Frates MC, Doubilet PM, et al. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med. 2003; 138:315–318.18. Erdogan MF, Gursoy A, Erdogan G. Natural course of benign thyroid nodules in a moderately iodine-deficient area. Clin Endocrinol (Oxf). 2006; 65:767–777.19. Ajmal S, Rapoport S, Ramirez Batlle H, Mazzaglia PJ. The natural history of the benign thyroid nodule: what is the appropriate follow-up strategy? J Am Coll Surg. 2015; 220:987–992.20. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.21. Na DG, Kim JH, Kim DS, Kim SJ. Thyroid nodules with minimal cystic changes have a low risk of malignancy. Ultrasonography. 2016; 35:153–158.22. Salmaslioğlu A, Erbil Y, Dural C, Isşsever H, Kapran Y, Ozarmağan S, et al. Predictive value of sonographic features in preoperative evaluation of malignant thyroid nodules in a multinodular goiter. World J Surg. 2008; 32:1948–1954.23. Henrichsen TL, Reading CC, Charboneau JW, Donovan DJ, Sebo TJ, Hay ID. Cystic change in thyroid carcinoma: prevalence and estimated volume in 360 carcinomas. J Clin Ultrasound. 2010; 38:361–366.24. Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011; 260:892–899.25. Na DG, Baek JH, Sung JY, Kim JH, Kim JK, Choi YJ, et al. Thyroid imaging reporting and data system risk stratification of thyroid nodules: categorization based on solidity and echogenicity. Thyroid. 2016; 26:562–572.26. Lee MJ, Kim EK, Kwak JY, Kim MJ. Partially cystic thyroid nodules on ultrasound: probability of malignancy and sonographic differentiation. Thyroid. 2009; 19:341–346.27. Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008; 247:762–770.28. Bonavita JA, Mayo J, Babb J, Bennett G, Oweity T, Macari M, et al. Pattern recognition of benign nodules at ultrasound of the thyroid: which nodules can be left alone? AJR Am J Roentgenol. 2009; 193:207–213.29. Moon WJ, Kwag HJ, Na DG. Are there any specific ultrasound findings of nodular hyperplasia ("leave me alone" lesion) to differentiate it from follicular adenoma? Acta Radiol. 2009; 50:383–388.30. Kim JY, Jung SL, Kim MK, Kim TJ, Byun JY. Differentiation of benign and malignant thyroid nodules based on the proportion of sponge-like areas on ultrasonography: imaging-pathologic correlation. Ultrasonography. 2015; 34:304–311.31. Kobayashi K, Hirokawa M, Yabuta T, Fukushima M, Kihara M, Takamura Y, et al. Papillary thyroid carcinoma with honeycomb-like multiple small cysts: characteristic features on ultrasonography. Eur Thyroid J. 2013; 2:270–274.32. Cappelli C, Castellano M, Pirola I, Cumetti D, Agosti B, Gandossi E, et al. The predictive value of ultrasound findings in the management of thyroid nodules. QJM. 2007; 100:29–35.33. Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002; 178:687–691.34. Alexander EK, Marqusee E, Orcutt J, Benson CB, Frates MC, Doubilet PM, et al. Thyroid nodule shape and prediction of malignancy. Thyroid. 2004; 14:953–958.35. Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995; 196:123–134.36. Jeh SK, Jung SL, Kim BS, Lee YS. Evaluating the degree of conformity of papillary carcinoma and follicular carcinoma to the reported ultrasonographic findings of malignant thyroid tumor. Korean J Radiol. 2007; 8:192–197.37. Yoon JH, Kim EK, Hong SW, Kwak JY, Kim MJ. Sonographic features of the follicular variant of papillary thyroid carcinoma. J Ultrasound Med. 2008; 27:1431–1437.38. Kim DS, Kim JH, Na DG, Park SH, Kim E, Chang KH, et al. Sonographic features of follicular variant papillary thyroid carcinomas in comparison with conventional papillary thyroid carcinomas. J Ultrasound Med. 2009; 28:1685–1692.39. Kwak JY, Jung I, Baek JH, Baek SM, Choi N, Choi YJ, et al. Image reporting and characterization system for ultrasound features of thyroid nodules: multicentric Korean retrospective study. Korean J Radiol. 2013; 14:110–117.40. Seo H, Na DG, Kim JH, Kim KW, Yoon JW. Ultrasound-based risk stratification for malignancy in thyroid nodules: a four-tier categorization system. Eur Radiol. 2015; 25:2153–2162.41. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005; 237:794–800.42. Andrioli M, Carzaniga C, Persani L. Standardized ultrasound report for thyroid nodules: the endocrinologist's viewpoint. Eur Thyroid J. 2013; 2:37–48.43. Su HK, Dos Reis LL, Lupo MA, Milas M, Orloff LA, Langer JE, et al. Striving toward standardization of reporting of ultrasound features of thyroid nodules and lymph nodes: a multidisciplinary consensus statement. Thyroid. 2014; 24:1341–1349.44. Grant EG, Tessler FN, Hoang JK, Langer JE, Beland MD, Berland LL, et al. Thyroid ultrasound reporting lexicon: white paper of the ACR thyroid imaging, reporting and data system (TIRADS) committee. J Am Coll Radiol. 2015; 12(12 Pt A):1272–1279.45. Langer JE, Khan A, Nisenbaum HL, Baloch ZW, Horii SC, Coleman BG, et al. Sonographic appearance of focal thyroiditis. AJR Am J Roentgenol. 2001; 176:751–754.46. Frates MC, Marqusee E, Benson CB, Alexander EK. Subacute granulomatous (de Quervain) thyroiditis: grayscale and color Doppler sonographic characteristics. J Ultrasound Med. 2013; 32:505–511.47. Scheible W, Leopold GR, Woo VL, Gosink BB. High-resolution real-time ultrasonography of thyroid nodules. Radiology. 1979; 133:413–417.48. Propper RA, Skolnick ML, Weinstein BJ, Dekker A. The nonspecificity of the thyroid halo sign. J Clin Ultrasound. 1980; 8:129–132.49. Lu C, Chang TC, Hsiao YL, Kuo MS. Ultrasonographic findings of papillary thyroid carcinoma and their relation to pathologic changes. J Formos Med Assoc. 1994; 93:933–938.50. Haber RS. Role of ultrasonography in the diagnosis and management of thyroid cancer. Endocr Pract. 2000; 6:396–400.51. Chan BK, Desser TS, McDougall IR, Weigel RJ, Jeffrey RB Jr. Common and uncommon sonographic features of papillary thyroid carcinoma. J Ultrasound Med. 2003; 22:1083–1090.52. Seo HS, Lee DH, Park SH, Min HS, Na DG. Thyroid follicular neoplasms: can sonography distinguish between adenomas and carcinomas? J Clin Ultrasound. 2009; 37:493–500.53. Brito JP, Gionfriddo MR, Al Nofal A, Boehmer KR, Leppin AL, Reading C, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. 2014; 99:1253–1263.54. Remonti LR, Kramer CK, Leitão CB, Pinto LC, Gross JL. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid. 2015; 25:538–550.55. Ahuja A, Chick W, King W, Metreweli C. Clinical significance of the comet-tail artifact in thyroid ultrasound. J Clin Ultrasound. 1996; 24:129–133.56. Beland MD, Kwon L, Delellis RA, Cronan JJ, Grant EG. Nonshadowing echogenic foci in thyroid nodules: are certain appearances enough to avoid thyroid biopsy? J Ultrasound Med. 2011; 30:753–760.57. Malhi H, Beland MD, Cen SY, Allgood E, Daley K, Martin SE, et al. Echogenic foci in thyroid nodules: significance of posterior acoustic artifacts. AJR Am J Roentgenol. 2014; 203:1310–1316.58. Popowicz B, Klencki M, Lewin´ski A, Słowin´ska-Klencka D. The usefulness of sonographic features in selection of thyroid nodules for biopsy in relation to the nodule's size. Eur J Endocrinol. 2009; 161:103–111.59. Lu Z, Mu Y, Zhu H, Luo Y, Kong Q, Dou J, et al. Clinical value of using ultrasound to assess calcification patterns in thyroid nodules. World J Surg. 2011; 35:122–127.60. Na DG, Kim DS, Kim SJ, Ryoo JW, Jung SL. Thyroid nodules with isolated macrocalcification: malignancy risk and diagnostic efficacy of fine-needle aspiration and core needle biopsy. Ultrasonography. 2015; 12. 27. [Epub]. http://dx.doi.org/10.14366/usg.15074.61. Kim BM, Kim MJ, Kim EK, Kwak JY, Hong SW, Son EJ, et al. Sonographic differentiation of thyroid nodules with eggshell calcifications. J Ultrasound Med. 2008; 27:1425–1430.62. Park M, Shin JH, Han BK, Ko EY, Hwang HS, Kang SS, et al. Sonography of thyroid nodules with peripheral calcifications. J Clin Ultrasound. 2009; 37:324–328.63. Rago T, Vitti P, Chiovato L, Mazzeo S, De Liperi A, Miccoli P, et al. Role of conventional ultrasonography and color flow-doppler sonography in predicting malignancy in 'cold' thyroid nodules. Eur J Endocrinol. 1998; 138:41–46.64. Frates MC, Benson CB, Doubilet PM, Cibas ES, Marqusee E. Can color Doppler sonography aid in the prediction of malignancy of thyroid nodules? J Ultrasound Med. 2003; 22:127–131.65. Appetecchia M, Solivetti FM. The association of colour flow Doppler sonography and conventional ultrasonography improves the diagnosis of thyroid carcinoma. Horm Res. 2006; 66:249–256.66. Ma JJ, Ding H, Xu BH, Xu C, Song LJ, Huang BJ, et al. Diagnostic performances of various gray-scale, color Doppler, and contrast-enhanced ultrasonography findings in predicting malignant thyroid nodules. Thyroid. 2014; 24:355–363.67. Moon HJ, Kwak JY, Kim MJ, Son EJ, Kim EK. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology. 2010; 255:260–269.68. Zhou JQ, Zhou C, Zhan WW, Zhou W, Dong YJ. Maximal, minimal, and mean pulsed Doppler parameters: which should be utilized in the diagnosis of thyroid nodules? Clin Radiol. 2014; 69:e477–e484.69. Fukunari N, Nagahama M, Sugino K, Mimura T, Ito K, Ito K. Clinical evaluation of color Doppler imaging for the differential diagnosis of thyroid follicular lesions. World J Surg. 2004; 28:1261–1265.70. Miyakawa M, Onoda N, Etoh M, Fukuda I, Takano K, Okamoto T, et al. Diagnosis of thyroid follicular carcinoma by the vascular pattern and velocimetric parameters using high resolution pulsed and power Doppler ultrasonography. Endocr J. 2005; 52:207–212.71. De Nicola H, Szejnfeld J, Logullo AF, Wolosker AM, Souza LR, Chiferi V Jr. Flow pattern and vascular resistive index as predictors of malignancy risk in thyroid follicular neoplasms. J Ultrasound Med. 2005; 24:897–904.72. Iared W, Shigueoka DC, Cristófoli JC, Andriolo R, Atallah AN, Ajzen SA, et al. Use of color Doppler ultrasonography for the prediction of malignancy in follicular thyroid neoplasms: systematic review and meta-analysis. J Ultrasound Med. 2010; 29:419–425.73. Choi YJ, Yun JS, Kim DH. Clinical and ultrasound features of cytology diagnosed follicular neoplasm. Endocr J. 2009; 56:383–389.74. Trimboli P, Sorrenti S. Low value of color flow-doppler in predicting malignancy of thyroid follicular neoplasms. Diagn Cytopathol. 2009; 37:391–392.75. Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015; 41:1126–1147.76. Kwak JY, Kim EK. Ultrasound elastography for thyroid nodules: recent advances. Ultrasonography. 2014; 33:75–82.77. Rago T, Santini F, Scutari M, Pinchera A, Vitti P. Elastography: new developments in ultrasound for predicting malignancy in thyroid nodules. J Clin Endocrinol Metab. 2007; 92:2917–2922.78. Asteria C, Giovanardi A, Pizzocaro A, Cozzaglio L, Morabito A, Somalvico F, et al. US-elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid. 2008; 18:523–531.79. Moon HJ, Sung JM, Kim EK, Yoon JH, Youk JH, Kwak JY. Diagnostic performance of gray-scale US and elastography in solid thyroid nodules. Radiology. 2012; 262:1002–1013.80. Cappelli C, Pirola I, Gandossi E, Agosti B, Cimino E, Casella C, et al. Real-time elastography: a useful tool for predicting malignancy in thyroid nodules with nondiagnostic cytologic findings. J Ultrasound Med. 2012; 31:1777–1782.81. Nell S, Kist JW, Debray TP, de Keizer B, van Oostenbrugge TJ, Borel Rinkes IH, et al. Qualitative elastography can replace thyroid nodule fine-needle aspiration in patients with soft thyroid nodules. A systematic review and meta-analysis. Eur J Radiol. 2015; 84:652–666.82. Rago T, Scutari M, Santini F, Loiacono V, Piaggi P, Di Coscio G, et al. Real-time elastosonography: useful tool for refining the presurgical diagnosis in thyroid nodules with indeterminate or nondiagnostic cytology. J Clin Endocrinol Metab. 2010; 95:5274–5280.83. Choi WJ, Park JS, Koo HR, Kim SY, Chung MS, Tae K. Ultrasound elastography using carotid artery pulsation in the differential diagnosis of sonographically indeterminate thyroid nodules. AJR Am J Roentgenol. 2015; 204:396–401.84. Samir AE, Dhyani M, Anvari A, Prescott J, Halpern EF, Faquin WC, et al. Shear-wave elastography for the preoperative risk stratification of follicular-patterned lesions of the thyroid: diagnostic accuracy and optimal measurement plane. Radiology. 2015; 277:565–573.85. Campanella P, Ianni F, Rota CA, Corsello SM, Pontecorvi A. Quantification of cancer risk of each clinical and ultrasonographic suspicious feature of thyroid nodules: a systematic review and meta-analysis. Eur J Endocrinol. 2014; 170:R203–R211.86. Horvath E, Majlis S, Rossi R, Franco C, Niedmann JP, Castro A, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009; 94:1748–1751.87. Russ G, Royer B, Bigorgne C, Rouxel A, Bienvenu-Perrard M, Leenhardt L. Prospective evaluation of thyroid imaging reporting and data system on 4550 nodules with and without elastography. Eur J Endocrinol. 2013; 168:649–655.88. Park JY, Lee HJ, Jang HW, Kim HK, Yi JH, Lee W, et al. A proposal for a thyroid imaging reporting and data system for ultrasound features of thyroid carcinoma. Thyroid. 2009; 19:1257–1264.89. Kim DW, Lee EJ, In HS, Kim SJ. Sonographic differentiation of partially cystic thyroid nodules: a prospective study. AJNR Am J Neuroradiol. 2010; 31:1961–1966.90. Park JM, Choi Y, Kwag HJ. Partially cystic thyroid nodules: ultrasound findings of malignancy. Korean J Radiol. 2012; 13:530–535.91. Vera MI, Meroño T, Urrutia MA, Parisi C, Morosan Y, Rosmarin M, et al. Differential profile of ultrasound findings associated with malignancy in mixed and solid thyroid nodules in an elderly female population. J Thyroid Res. 2014; 2014:761653.92. Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract. 2010; 16:Suppl 1. 1–43.93. Wémeau JL, Sadoul JL, d'Herbomez M, Monpeyssen H, Tramalloni J, Leteurtre E, et al. Guidelines of the French society of endocrinology for the management of thyroid nodules. Ann Endocrinol (Paris). 2011; 72:251–228.94. Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014; 81:Suppl 1. 1–122.95. National Comprehensive Cancer Network. 2014 Practice Guidelines in Oncology-Thyroid Carcinoma v.2. Web site. Accessed May 18, 2015. http://www.nccn.org/.96. Andersen PE, Kinsella J, Loree TR, Shaha AR, Shah JP. Differentiated carcinoma of the thyroid with extrathyroidal extension. Am J Surg. 1995; 170:467–470.97. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: massive but not minimal extension affects the relapse-free survival. World J Surg. 2006; 30:780–786.98. Riemann B, Krämer JA, Schmid KW, Dralle H, Dietlein M, Schicha H, et al. Risk stratification of patients with locally aggressive differentiated thyroid cancer. Results of the MSDS trial. Nuklearmedizin. 2010; 49:79–78.99. Radowsky JS, Howard RS, Burch HB, Stojadinovic A. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid. 2014; 24:241–244.100. Lee CY, Kim SJ, Ko KR, Chung KW, Lee JH. Predictive factors for extrathyroidal extension of papillary thyroid carcinoma based on preoperative sonography. J Ultrasound Med. 2014; 33:231–238.101. Kwak JY, Kim EK, Youk JH, Kim MJ, Son EJ, Choi SH, et al. Extrathyroid extension of well-differentiated papillary thyroid microcarcinoma on US. Thyroid. 2008; 18:609–614.102. Shimamoto K, Satake H, Sawaki A, Ishigaki T, Funahashi H, Imai T. Preoperative staging of thyroid papillary carcinoma with ultrasonography. Eur J Radiol. 1998; 29:4–10.103. Choi JS, Chung WY, Kwak JY, Moon HJ, Kim MJ, Kim EK. Staging of papillary thyroid carcinoma with ultrasonography: performance in a large series. Ann Surg Oncol. 2011; 18:3572–3578.104. Park JS, Son KR, Na DG, Kim E, Kim S. Performance of preoperative sonographic staging of papillary thyroid carcinoma based on the sixth edition of the AJCC/UICC TNM classification system. AJR Am J Roentgenol. 2009; 192:66–72.105. Moon SJ, Kim DW, Kim SJ, Ha TK, Park HK, Jung SJ. Ultrasound assessment of degrees of extrathyroidal extension in papillary thyroid microcarcinoma. Endocr Pract. 2014; 20:1037–1043.106. Kim SS, Lee BJ, Lee JC, Kim SJ, Lee SH, Jeon YK, et al. Preoperative ultrasonographic tumor characteristics as a predictive factor of tumor stage in papillary thyroid carcinoma. Head Neck. 2011; 33:1719–1726.107. Ito Y, Miyauchi A, Oda H, Kobayashi K, Kihara M, Miya A. Revisiting low-risk thyroid papillary microcarcinomas resected without observation: was immediate surgery necessary? World J Surg. 2016; 40:523–528.108. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97:418–428.109. Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005; 103:2269–2273.110. Koo JH, Shin JH, Han BK, Ko EY, Kang SS. Cystic thyroid nodules after aspiration mimicking malignancy: sonographic characteristics. J Ultrasound Med. 2010; 29:1415–1421.111. Park NH, Kim DW, Park HJ, Lee EJ, Park JS, Park SI, et al. Thyroid cysts treated with ethanol ablation can mimic malignancy during sonographic follow-up. J Clin Ultrasound. 2011; 39:441–446.112. Zacharia TT, Perumpallichira JJ, Sindhwani V, Chavhan G. Gray-scale and color Doppler sonographic findings in a case of subacute granulomatous thyroiditis mimicking thyroid carcinoma. J Clin Ultrasound. 2002; 30:442–444.113. Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010; 34:28–35.114. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014; 24:27–34.115. Pacini F. Management of papillary thyroid microcarcinoma: primum non nocere. J Clin Endocrinol Metab. 2013; 98:1391–1393.116. Takami H, Ito Y, Okamoto T, Onoda N, Noguchi H, Yoshida A. Revisiting the guidelines issued by the Japanese Society of Thyroid Surgeons and Japan Association of Endocrine Surgeons: a gradual move towards consensus between Japanese and western practice in the management of thyroid carcinoma. World J Surg. 2014; 38:2002–2010.117. Oda H, Miyauchi A, Ito Y, Yoshioka K, Nakayama A, Sasai H, et al. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid. 2016; 26:150–155.118. Brito JP, Ito Y, Miyauchi A, Tuttle RM. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid. 2016; 26:144–149.119. Ghossein R, Ganly I, Biagini A, Robenshtok E, Rivera M, Tuttle RM. Prognostic factors in papillary microcarcinoma with emphasis on histologic subtyping: a clinicopathologic study of 148 cases. Thyroid. 2014; 24:245–253.120. Lecumberri B, Alvarez-Escolá C, Martín-Vaquero P, Nistal M, Martín V, Riesco-Eizaguirre G, et al. Solitary hemorrhagic cerebellar metastasis from occult papillary thyroid microcarcinoma. Thyroid. 2010; 20:563–567.121. Jeon MJ, Kim WG, Choi YM, Kwon H, Lee YM, Sung TY, et al. Features predictive of distant metastasis in papillary thyroid microcarcinomas. Thyroid. 2016; 26:161–168.122. Noguchi S, Yamashita H, Uchino S, Watanabe S. Papillary microcarcinoma. World J Surg. 2008; 32:747–753.123. Roti E, Rossi R, Trasforini G, Bertelli F, Ambrosio MR, Busutti L, et al. Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab. 2006; 91:2171–2178.124. Mercante G, Frasoldati A, Pedroni C, Formisano D, Renna L, Piana S, et al. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid. 2009; 19:707–716.125. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009; 19:1159–1165.126. Moon HJ, Kim EK, Yoon JH, Kwak JY. Malignancy risk stratification in thyroid nodules with nondiagnostic results at cytologic examination: combination of thyroid imaging reporting and data system and the Bethesda System. Radiology. 2015; 274:287–295.127. Kim SY, Han KH, Moon HJ, Kwak JY, Chung WY, Kim EK. Thyroid nodules with benign findings at cytologic examination: results of long-term follow-up with US. Radiology. 2014; 271:272–281.128. Rosario PW. Thyroid nodules with atypia or follicular lesions of undetermined significance (Bethesda Category III): importance of ultrasonography and cytological subcategory. Thyroid. 2014; 24:1115–1120.129. Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol. 2012; 56:333–339.130. Singh RS, Wang HH. Timing of repeat thyroid fine-needle aspiration in the management of thyroid nodules. Acta Cytol. 2011; 55:544–548.131. Lubitz CC, Nagarkatti SS, Faquin WC, Samir AE, Hassan MC, Barbesino G, et al. Diagnostic yield of nondiagnostic thyroid nodules is not altered by timing of repeat biopsy. Thyroid. 2012; 22:590–594.132. Yeon JS, Baek JH, Lim HK, Ha EJ, Kim JK, Song DE, et al. Thyroid nodules with initially nondiagnostic cytologic results: the role of core-needle biopsy. Radiology. 2013; 268:274–280.133. Samir AE, Vij A, Seale MK, Desai G, Halpern E, Faquin WC, et al. Ultrasound-guided percutaneous thyroid nodule core biopsy: clinical utility in patients with prior nondiagnostic fine-needle aspirate. Thyroid. 2012; 22:461–467.134. Na DG, Kim JH, Sung JY, Baek JH, Jung KC, Lee H, et al. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid. 2012; 22:468–475.135. Choi SH, Baek JH, Lee JH, Choi YJ, Hong MJ, Song DE, et al. Thyroid nodules with initially non-diagnostic, fine-needle aspiration results: comparison of core-needle biopsy and repeated fine-needle aspiration. Eur Radiol. 2014; 24:2819–2826.136. Ha EJ, Baek JH, Lee JH, Song DE, Kim JK, Shong YK, et al. Sonographically suspicious thyroid nodules with initially benign cytologic results: the role of a core needle biopsy. Thyroid. 2013; 23:703–708.137. Kwak JY, Kim EK, Kim HJ, Kim MJ, Son EJ, Moon HJ. How to combine ultrasound and cytological information in decision making about thyroid nodules. Eur Radiol. 2009; 19:1923–1931.138. Kwak JY, Koo H, Youk JH, Kim MJ, Moon HJ, Son EJ, et al. Value of US correlation of a thyroid nodule with initially benign cytologic results. Radiology. 2010; 254:292–300.139. Chernyavsky VS, Shanker BA, Davidov T, Crystal JS, Eng O, Ibrahim K, et al. Is one benign fine needle aspiration enough? Ann Surg Oncol. 2012; 19:1472–1476.140. Shin JH, Han BK, Ko K, Choe YH, Oh YL. Value of repeat ultrasound-guided fine-needle aspiration in nodules with benign cytological diagnosis. Acta Radiol. 2006; 47:469–473.141. Hwang SH, Sung JM, Kim EK, Moon HJ, Kwak JY. Imaging-cytology correlation of thyroid nodules with initially benign cytology. Int J Endocrinol. 2014; 2014:491508.142. Moon HJ, Kim EK, Kwak JY. Malignancy risk stratification in thyroid nodules with benign results on cytology: combination of thyroid imaging reporting and data system and Bethesda system. Ann Surg Oncol. 2014; 21:1898–1903.143. Rosário PW, Calsolari MR. What is the best criterion for repetition of fine-needle aspiration in thyroid nodules with initially benign cytology? Thyroid. 2015; 25:1115–1120.144. Chehade JM, Silverberg AB, Kim J, Case C, Mooradian AD. Role of repeated fine-needle aspiration of thyroid nodules with benign cytologic features. Endocr Pract. 2001; 7:237–243.145. Orlandi A, Puscar A, Capriata E, Fideleff H. Repeated fine-needle aspiration of the thyroid in benign nodular thyroid disease: critical evaluation of long-term follow-up. Thyroid. 2005; 15:274–278.146. Pinchot SN, Al-Wagih H, Schaefer S, Sippel R, Chen H. Accuracy of fine-needle aspiration biopsy for predicting neoplasm or carcinoma in thyroid nodules 4 cm or larger. Arch Surg. 2009; 144:649–655.147. Carrillo JF, Frias-Mendivil M, Ochoa-Carrillo FJ, Ibarra M. Accuracy of fine-needle aspiration biopsy of the thyroid combined with an evaluation of clinical and radiologic factors. Otolaryngol Head Neck Surg. 2000; 122:917–921.148. Albuja-Cruz MB, Goldfarb M, Gondek SS, Allan BJ, Lew JI. Reliability of fine-needle aspiration for thyroid nodules greater than or equal to 4 cm. J Surg Res. 2013; 181:6–10.149. Porterfield JR Jr, Grant CS, Dean DS, Thompson GB, Farley DR, Richards ML, et al. Reliability of benign fine needle aspiration cytology of large thyroid nodules. Surgery. 2008; 144:963–968.150. Kuru B, Gulcelik NE, Gulcelik MA, Dincer H. The false-negative rate of fine-needle aspiration cytology for diagnosing thyroid carcinoma in thyroid nodules. Langenbecks Arch Surg. 2010; 395:127–132.151. Yoon JH, Kwak JY, Moon HJ, Kim MJ, Kim EK. The diagnostic accuracy of ultrasound-guided fine-needle aspiration biopsy and the sonographic differences between benign and malignant thyroid nodules 3 cm or larger. Thyroid. 2011; 21:993–1000.152. Nou E, Kwong N, Alexander LK, Cibas ES, Marqusee E, Alexander EK. Determination of the optimal time interval for repeat evaluation after a benign thyroid nodule aspiration. J Clin Endocrinol Metab. 2014; 99:510–516.153. Nakamura H, Hirokawa M, Ota H, Kihara M, Miya A, Miyauchi A. Is an increase in thyroid nodule volume a risk factor for malignancy? Thyroid. 2015; 25:804–811.154. Ho AS, Sarti EE, Jain KS, Wang H, Nixon IJ, Shaha AR, et al. Malignancy rate in thyroid nodules classified as Bethesda category III (AUS/FLUS). Thyroid. 2014; 24:832–839.155. Faquin WC, Baloch ZW. Fine-needle aspiration of follicular patterned lesions of the thyroid: Diagnosis, management, and follow-up according to National Cancer Institute (NCI) recommendations. Diagn Cytopathol. 2010; 38:731–739.156. Kim DW, Lee EJ, Jung SJ, Ryu JH, Kim YM. Role of sonographic diagnosis in managing Bethesda class III nodules. AJNR Am J Neuroradiol. 2011; 32:2136–2141.157. Gweon HM, Son EJ, Youk JH, Kim JA. Thyroid nodules with Bethesda system III cytology: can ultrasonography guide the next step? Ann Surg Oncol. 2013; 20:3083–3088.158. Yoo WS, Choi HS, Cho SW, Moon JH, Kim KW, Park HJ, et al. The role of ultrasound findings in the management of thyroid nodules with atypia or follicular lesions of undetermined significance. Clin Endocrinol (Oxf). 2014; 80:735–774.159. Jeong SH, Hong HS, Lee EH, Cha JG, Park JS, Kwak JJ. Outcome of thyroid nodules characterized as atypia of undetermined significance or follicular lesion of undetermined significance and correlation with Ultrasound features and BRAF(V600E) mutation analysis. AJR Am J Roentgenol. 2013; 201:W854–W860.160. Bongiovanni M, Krane JF, Cibas ES, Faquin WC. The atypical thyroid fine-needle aspiration: past, present, and future. Cancer Cytopathol. 2012; 120:73–86.161. Hyeon J, Ahn S, Shin JH, Oh YL. The prediction of malignant risk in the category "atypia of undetermined significance/follicular lesion of undetermined significance" of the Bethesda System for Reporting Thyroid Cytopathology using subcategorization and BRAF mutation results. Cancer Cytopathol. 2014; 122:368–376.162. Sullivan PS, Hirschowitz SL, Fung PC, Apple SK. The impact of atypia/follicular lesion of undetermined significance and repeat fine-needle aspiration: 5 years before and after implementation of the Bethesda System. Cancer Cytopathol. 2014; 122:866–872.163. Renshaw AA. Does a repeated benign aspirate change the risk of malignancy after an initial atypical thyroid fine-needle aspiration? Am J Clin Pathol. 2010; 134:788–792.164. VanderLaan PA, Marqusee E, Krane JF. Clinical outcome for atypia of undetermined significance in thyroid fine-needle aspirations: should repeated fna be the preferred initial approach? Am J Clin Pathol. 2011; 135:770–775.165. Wu HH, Inman A, Cramer HM. Subclassification of "atypia of undetermined significance" in thyroid fine-needle aspirates. Diagn Cytopathol. 2014; 42:23–29.166. Park KT, Ahn SH, Mo JH, Park YJ, Park do J, Choi SI, et al. Role of core needle biopsy and ultrasonographic finding in management of indeterminate thyroid nodules. Head Neck. 2011; 33:160–165.167. Hahn SY, Shin JH, Han BK, Ko EY, Ko ES. Ultrasonography-guided core needle biopsy for the thyroid nodule: does the procedure hold any benefit for the diagnosis when fine-needle aspiration cytology analysis shows inconclusive results? Br J Radiol. 2013; 86:20130007.168. Lee KH, Shin JH, Oh YL, Hahn SY. Atypia of undetermined significance in thyroid fine-needle aspiration cytology: prediction of malignancy by US and comparison of methods for further management. Ann Surg Oncol. 2014; 21:2326–2331.169. Na DG, Min HS, Lee H, Won JK, Seo HB, Kim JH. Role of core needle biopsy in the management of atypia/follicular lesion of undetermined significance thyroid nodules: comparison with repeat fine-needle aspiration in subcategory nodules. Eur Thyroid J. 2015; 4:189–196.170. Bernet V, Hupart KH, Parangi S, Woeber KA. AACE/ACE disease state commentary: molecular diagnostic testing of thyroid nodules with indeterminate cytopathology. Endocr Pract. 2014; 20:360–363.171. Ferris RL, Baloch Z, Bernet V, Chen A, Fahey TJ 3rd, Ganly I, et al. American thyroid association statement on surgical application of molecular profiling for thyroid nodules: current impact on perioperative decision making. Thyroid. 2015; 25:760–768.172. Kwak JY, Kim EK, Kim MJ, Hong SW, Choi SH, Son EJ, et al. The role of ultrasound in thyroid nodules with a cytology reading of "suspicious for papillary thyroid carcinoma". Thyroid. 2008; 18:517–522.173. Mulla M, Schulte KM. Central cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the central compartment. Clin Endocrinol (Oxf). 2012; 76:131–113.174. Rotstein L. The role of lymphadenectomy in the management of papillary carcinoma of the thyroid. J Surg Oncol. 2009; 99:186–188.175. Sivanandan R, Soo KC. Pattern of cervical lymph node metastases from papillary carcinoma of the thyroid. Br J Surg. 2001; 88:1241–1244.176. Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012; 22:1144–1152.177. Amdur RJ, Mazzaferri EL. Essentials of Thyroid Cancer Management. New York: Springer;2005. p. 212–215.178. Spate VL, Morris JS, Nichols TA, Baskett CK, Mason MM, Horsman TL, et al. Longitudinal study of iodine in toenails following IV administration of an iodine containing contrast agent. J Radioanal Nucl Chem. 1998; 236:71–76.179. Padovani RP, Kasamatsu TS, Nakabashi CC, Camacho CP, Andreoni DM, Malouf EZ, et al. One month is sufficient for urinary iodine to return to its baseline value after the use of water-soluble iodinated contrast agents in post-thyroidectomy patients requiring radioiodine therapy. Thyroid. 2012; 22:926–930.180. Sohn SY, Choi JH, Kim NK, Joung JY, Cho YY, Park SM, et al. The impact of iodinated contrast agent administered during preoperative computed tomography scan on body iodine pool in patients with differentiated thyroid cancer preparing for radioactive iodine treatment. Thyroid. 2014; 24:872–877.181. Ho JD, Tsang JF, Scoggan KA, Leslie WD. Urinary iodine clearance following iodinated contrast administration: a comparison of euthyroid and postthyroidectomy subjects. J Thyroid Res. 2014; 2014:580569.182. Mishra A, Pradhan PK, Gambhir S, Sabaretnam M, Gupta A, Babu S. Preoperative contrast-enhanced computerized tomography should not delay radioiodine ablation in differentiated thyroid carcinoma patients. J Surg Res. 2015; 193:731–737.183. Tala Jury HP, Castagna MG, Fioravanti C, Cipri C, Brianzoni E, Pacini F. Lack of association between urinary iodine excretion and successful thyroid ablation in thyroid cancer patients. J Clin Endocrinol Metab. 2010; 95:230–237.184. Kim E, Park JS, Son KR, Kim JH, Jeon SJ, Na DG. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid. 2008; 18:411–418.185. Ahn JE, Lee JH, Yi JS, Shong YK, Hong SJ, Lee DH, et al. Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J Surg. 2008; 32:1552–1558.186. Lesnik D, Cunnane ME, Zurakowski D, Acar GO, Ecevit C, Mace A, et al. Papillary thyroid carcinoma nodal surgery directed by a preoperative radiographic map utilizing CT scan and ultrasound in all primary and reoperative patients. Head Neck. 2014; 36:191–202.187. Lee DW, Ji YB, Sung ES, Park JS, Lee YJ, Park DW, et al. Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol. 2013; 39:191–196.188. Yeh MW, Bauer AJ, Bernet VA, Ferris RL, Loevner LA, Mandel SJ, et al. American Thyroid Association statement on preoperative imaging for thyroid cancer surgery. Thyroid. 2015; 25:3–14.189. Choi JS, Kim J, Kwak JY, Kim MJ, Chang HS, Kim EK. Preoperative staging of papillary thyroid carcinoma: comparison of ultrasound imaging and CT. AJR Am J Roentgenol. 2009; 193:871–878.190. Leenhardt L, Erdogan MF, Hegedus L, Mandel SJ, Paschke R, Rago T, et al. 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J. 2013; 2:147–159.191. Rosário PW, de Faria S, Bicalho L, Alves MF, Borges MA, Purisch S, et al. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J Ultrasound Med. 2005; 24:1385–1389.192. Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B, et al. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab. 2007; 92:3590–3594.193. Ito Y, Jikuzono T, Higashiyama T, Asahi S, Tomoda C, Takamura Y, et al. Clinical significance of lymph node metastasis of thyroid papillary carcinoma located in one lobe. World J Surg. 2006; 30:1821–1828.194. Sywak M, Cornford L, Roach P, Stalberg P, Sidhu S, Delbridge L. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery. 2006; 140:1000–1005.195. Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope. 2011; 121:487–491.196. Gemsenjäger E, Perren A, Seifert B, Schüler G, Schweizer I, Heitz PU. Lymph node surgery in papillary thyroid carcinoma. J Am Coll Surg. 2003; 197:182–190.197. Cranshaw IM, Carnaille B. Micrometastases in thyroid cancer. An important finding? Surg Oncol. 2008; 17:253–225.198. Bardet S, Malville E, Rame JP, Babin E, Samama G, De Raucourt D, et al. Macroscopic lymph-node involvement and neck dissection predict lymph-node recurrence in papillary thyroid carcinoma. Eur J Endocrinol. 2008; 158:551–560.199. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Preoperative ultrasonographic examination for lymph node metastasis: usefulness when designing lymph node dissection for papillary microcarcinoma of the thyroid. World J Surg. 2004; 28:498–501.200. Bardet S, Ciappuccini R, Quak E, Rame JP, Blanchard D, de Raucourt D, et al. Prognostic value of microscopic lymph node involvement in patients with papillary thyroid cancer. J Clin Endocrinol Metab. 2015; 100:132–140.201. Rondeau G, Fish S, Hann LE, Fagin JA, Tuttle RM. Ultrasonographically detected small thyroid bed nodules identified after total thyroidectomy for differentiated thyroid cancer seldom show clinically significant structural progression. Thyroid. 2011; 21:845–853.202. Robenshtok E, Fish S, Bach A, Domínguez JM, Shaha A, Tuttle RM. Suspicious cervical lymph nodes detected after thyroidectomy for papillary thyroid cancer usually remain stable over years in properly selected patients. J Clin Endocrinol Metab. 2012; 97:2706–2713.203. Na DG, Lee JH, Jung SL, Kim JH, Sung JY, Shin JH, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012; 13:117–125.204. Bennedbaek FN, Nielsen LK, Hegedüs L. Effect of percutaneous ethanol injection therapy versus suppressive doses of L-thyroxine on benign solitary solid cold thyroid nodules: a randomized trial. J Clin Endocrinol Metab. 1998; 83:830–835.205. Sung JY, Baek JH, Kim KS, Lee D, Yoo H, Kim JK, et al. Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology. 2013; 269:293–300.206. Sung JY, Kim YS, Choi H, Lee JH, Baek JH. Optimum first-line treatment technique for benign cystic thyroid nodules: ethanol ablation or radiofrequency ablation? AJR Am J Roentgenol. 2011; 196:W210–W214.207. Døssing H, Bennedbæk FN, Hegedüs L. Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur J Endocrinol. 2011; 165:123–128.208. Lim HK, Lee JH, Ha EJ, Sung JY, Kim JK, Baek JH. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013; 23:1044–1049.209. Sung JY, Baek JH, Jung SL, Kim JH, Kim KS, Lee D, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid. 2015; 25:112–117.210. Valcavi R, Riganti F, Bertani A, Formisano D, Pacella CM. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid. 2010; 20:1253–1261.211. Papini E, Rago T, Gambelunghe G, Valcavi R, Bizzarri G, Vitti P, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab. 2014; 99:3653–3365.212. Ha EJ, Baek JH, Kim KW, Pyo J, Lee JH, Baek SH, et al. Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and bayesian network meta-analysis. J Clin Endocrinol Metab. 2015; 100:1903–1911.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 2021 Korean Thyroid Imaging Reporting and Data System (2021-K-TIRADS) and Imaging-Based Management of Thyroid Nodules: Korean Society of Thyroid Radiology Consensus Statement and Recommendations
- Standardized Imaging and Reporting for Thyroid Ultrasound: Korean Society of Thyroid Radiology Consensus Statement and Recommendation
- Ultrasonography Diagnosis of Thyroid Nodules and Cervical Metastatic Lymph Nodes
- Clinical applications of Doppler ultrasonography for thyroid disease: consensus statement by the Korean Society of Thyroid Radiology
- Sonographic Evaluation of Thyroid Nodules