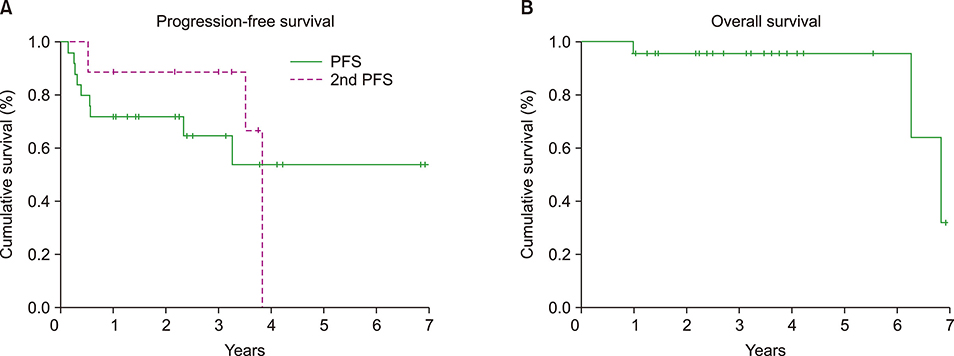
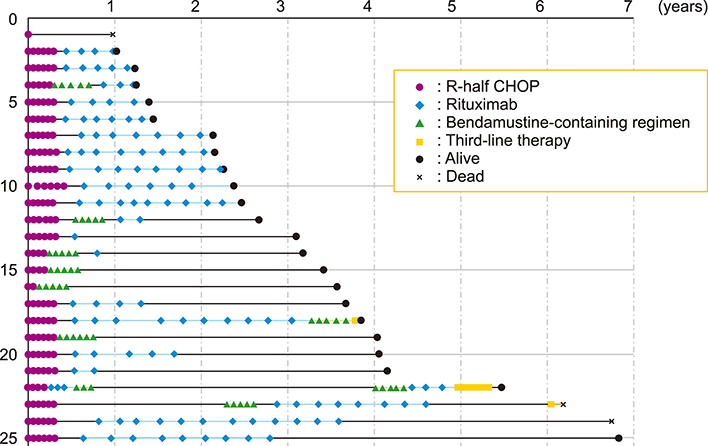
Blood Res.
2019 Jun;54(2):153-156. 10.5045/br.2019.54.2.153.
What is the most appropriate regimen for untreated Waldenström macroglobulinemia? - An updated analysis of rituximab and half-dose CHOP therapy and cost effectiveness
- Affiliations
-
- 1Hematology Division, National Hospital Organization Disaster Medical Center, Tokyo, Japan. nao26nao26@gmail.com
- 2Clinical Research Division, National Hospital Organization Disaster Medical Center, Tokyo, Japan.
- 3Pharmaceutical Division, National Hospital Organization Disaster Medical Center, Tokyo, Japan.
- 4Laboratory and Pathology Division, National Hospital Organization Disaster Medical Center, Tokyo, Japan.
- KMID: 2451019
- DOI: http://doi.org/10.5045/br.2019.54.2.153
Abstract
- No abstract available.
Figure
Cited by 1 articles
-
Successful treatment of non-IgM lymphoplasmacytic lymphoma by bortezomib-containing regimen: case reports and review of literature
Kenichi Ito, Risa Nishiyama, Kazuhiko Hirano, Kazuaki Yamada, Naohiro Sekiguchi
Blood Res. 2019;54(3):236-240. doi: 10.5045/br.2019.54.3.236.
Reference
-
1. Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström's macroglobulinemia. N Engl J Med. 2012; 367:826–833.
Article2. Sekiguchi N, Nomoto J, Nagata A, et al. Gene expression profile signature of aggressive Waldenström macroglobulinemia with chromosome 6q deletion. Biomed Res Int. 2018; 2018:6728128.
Article3. Sekiguchi N, Hamano A, Kitagawa T, et al. Impact of rituximab and half-dose CHOP as primary therapy for untreated symptomatic Waldenström Macroglobulinemia: review of a combined regimen of rituximab with an alkylating agent. Blood Res. 2018; 53:117–122.
Article4. Buske C, Hoster E, Dreyling M, et al. The addition of rituximab to front-line therapy with CHOP (R-CHOP) results in a higher response rate and longer time to treatment failure in patients with lymphoplasmacytic lymphoma: results of a randomized trial of the German Low-Grade Lymphoma Study Group (GLSG). Leukemia. 2009; 23:153–161.
Article5. Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013; 381:1203–1210.
Article6. Buske C, Sadullah S, Kastritis E, et al. Treatment and outcome patterns in European patients with Waldenström's macroglobulinaemia: a large, observational, retrospective chart review. Lancet Haematol. 2018; 5:e299–e309.
Article7. Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, et al. Primary treatment of Waldenström macroglobulinemia with dexamethasone, rituximab, and cyclophosphamide. J Clin Oncol. 2007; 25:3344–3349.
Article8. Olszewski AJ, Treon SP, Castillo JJ. Application and outcomes of bendamustine- or bortezomib-based therapy for Waldenstrom's macroglobulinemia. Blood (ASH Annual Meeting Abstracts). 2017; 130:Suppl. abst 348.9. Lee HS, Kim K, Yoon DH, et al. Clinical factors associated with response or survival after chemotherapy in patients with Waldenström macroglobulinemia in Korea. Biomed Res Int. 2014; 2014:253243.
Article10. Saito A, Isoda A, Kojima M, et al. Retrospective analysis of prognostic factors for WaldenstrXMLLink_XYZm macroglobulinemia: a multicenter cooperative study in Japan. Int J Hematol. 2017; 106:681–690.
Article11. Treon SP, Ioakimidis L, Soumerai JD, et al. Primary therapy of Waldenström macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG clinical trial 05-180. J Clin Oncol. 2009; 27:3830–3835.
Article12. Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström's macroglobulinemia. N Engl J Med. 2015; 372:1430–1440.
Article13. Dimopoulos MA, Tedeschi A, Trotman J, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenström's macroglobulinemia. N Engl J Med. 2018; 378:2399–2410.
Article14. Olszewski AJ, Castillo JJ. Ibrutinib and rituximab in Waldenström's macroglobulinemia. N Engl J Med. 2018; 379:1973–1974.
Article15. Aiello A, D'Ausilio A, Lo Muto R, Randon F, Laurenti L. Cost-effectiveness analysis of ibrutinib in patients with Waldenström macroglobulinemia in Italy. J Mark Access Health Policy. 2017; 5:1393308.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of rituximab and half-dose CHOP as primary therapy for untreated symptomatic Waldenström Macroglobulinemia: review of a combined regimen of rituximab with an alkylating agent
- Waldenström macroglobulinemia presenting with neurological symptoms after chemotherapy
- Utility Analysis for Pegfilgrastim in DLBCL Patients on R-CHOP Regimen
- A Case of Mantle Cell Lymphoma Treated with Autologous Stem Cell Transplantation and Rituximab
- A Case of Lymphoplasmacytic Lymphoma/Waldenström's Macroglobulinemia with IgM-κ and IgA-λ Biclonal Gammopathy