Yonsei Med J.
2019 Jun;60(6):570-577. 10.3349/ymj.2019.60.6.570.
Factors Associated with Adherence to Allergen Specific Subcutaneous Immunotherapy
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 2Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. drsys93@naver.com
- 3Department of Pulmonary, Allergy and Critical Care Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- KMID: 2446963
- DOI: http://doi.org/10.3349/ymj.2019.60.6.570
Abstract
- PURPOSE
Allergen-specific immunotherapy (AIT) is known to be the only therapeutic modality to alter the natural course of allergic diseases. However, at least 3 years of treatment is recommended for achieving long-term disease modifying effect. This study aimed to investigate factors associated with immunotherapy non-adherence in real practice.
MATERIALS AND METHODS
We retrospectively reviewed medical records of patients who were diagnosed with allergic rhinitis, asthma, or atopic dermatitis, and received AIT to common allergens such as house dust mite and/or pollens from January 2007 to August 2014. In this study, non-adherence was defined as not completing 3 years of AIT.
RESULTS
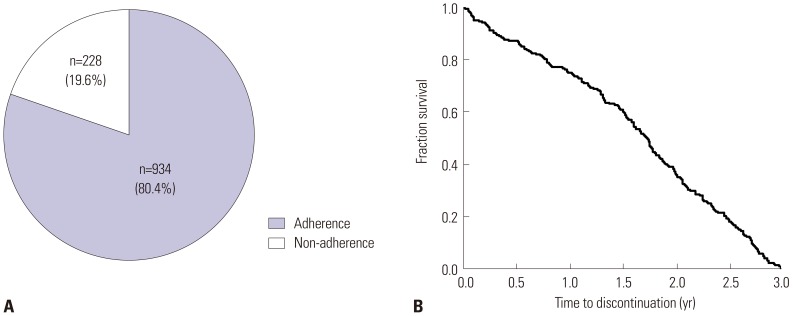
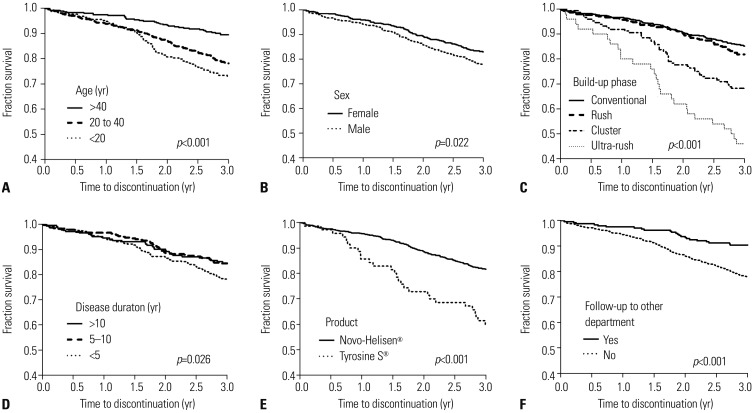
Among 1162 patients enrolled, 228 (19.6%) failed to complete 3 years of AIT. In multivariate analysis, age less than 20 years [odds ratio (OR) 3.11, 95% confidence interval (CI) 1.70-5.69] and 20 to 40 years (OR 2.01, 95% CI 1.17-3.43), cluster build-up (OR 1.78, 95% CI 1.05-3.02) and ultra-rush build-up schedules (OR 5.46, 95% CI 2.40-12.43), and absence of visit to other departments in the same hospital (OR 1.87, 95% CI 1.05-3.32) were independently associated with immunotherapy non-adherence. Disease duration of 5-10 years was negatively associated with non-adherence compared to shorter disease duration of less than 5 years (OR 0.61, 95% CI 0.40-0.94). Although male sex and commercial product of AIT, Tyrosine S®, compared to Novo-Helisen® were non-adherent factors in univariate analysis, no statistical significances were identified in multivariate analysis.
CONCLUSION
Various factors are associated with immunotherapy adherence affecting the utility of immunotherapy. Clinicians should be aware of factors associated with adherence to maximize the utility of allergen-specific subcutaneous immunotherapy.
MeSH Terms
Figure
Cited by 1 articles
-
Safety and Utility of Rush Immunotherapy with Aqueous Allergen Extracts for Treatment of Respiratory Allergies
Ji-Ho Lee, Jae-Hwa Choi, Keun-Bae Jeong, Seok Jeong Lee, Myoung Kyu Lee, Won-Yeon Lee, Suk Joong Yong, Sang-Ha Kim
J Korean Med Sci. 2020;36(3):e18. doi: 10.3346/jkms.2021.36.e18.
Reference
-
1. Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future. Allergy Asthma Immunol Res. 2016; 8:191–197. PMID: 26922928.
Article2. Roberts G, Pfaar O, Akdis CA, Ansotegui IJ, Durham SR, Gerth van Wijk R, et al. EAACI Guidelines on Allergen Immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018; 73:765–798. PMID: 28940458.3. Cox LS, Hankin C, Lockey R. Allergy immunotherapy adherence and delivery route: location does not matter. J Allergy Clin Immunol Pract. 2014; 2:156–160. PMID: 24607042.
Article4. Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008; CD000011. PMID: 18425859.
Article5. Passalacqua G, Baiardini I, Senna G, Canonica GW. Adherence to pharmacological treatment and specific immunotherapy in allergic rhinitis. Clin Exp Allergy. 2013; 43:22–28. PMID: 23278877.
Article6. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005; 353:487–497. PMID: 16079372.
Article7. Lower T, Henry J, Mandik L, Janosky J, Friday GA Jr. Compliance with allergen immunotherapy. Ann Allergy. 1993; 70:480–482. PMID: 8507043.8. Calderon MA, Cox L, Casale TB, Mösges R, Pfaar O, Malling HJ, et al. The effect of a new communication template on anticipated willingness to initiate or resume allergen immunotherapy: an internet-based patient survey. Allergy Asthma Clin Immunol. 2015; 11:17. PMID: 26015786.
Article9. Vita D, Caminiti L, Ruggeri P, Pajno GB. Sublingual immunotherapy: adherence based on timing and monitoring control visits. Allergy. 2010; 65:668–669. PMID: 19845569.
Article10. Lee JH, Kim SC, Choi H, Jung CG, Ban GY, Shin YS, et al. A Retrospective study of clinical response predictors in subcutaneous allergen immunotherapy with house dust mites for allergic rhinitis. Allergy Asthma Immunol Res. 2018; 10:18–24. PMID: 29178674.
Article11. Mahesh PA, Vedanthan PK, Amrutha DH, Giridhar BH, Prabhakar AK. Factors associated with non-adherence to specific allergen immunotherapy in management of respiratory allergy. Indian J Chest Dis Allied Sci. 2010; 52:91–95. PMID: 20578401.12. More DR, Hagan LL. Factors affecting compliance with allergen immunotherapy at a military medical center. Ann Allergy Asthma Immunol. 2002; 88:391–394. PMID: 11991556.
Article13. Rhodes BJ. Patient dropouts before completion of optimal dose, multiple allergen immunotherapy. Ann Allergy Asthma Immunol. 1999; 82:281–286. PMID: 10094219.
Article14. Guenechea-Sola M, Hariri SR, Galoosian A, Yusin JS. A retrospective review of veterans' adherence to allergen immunotherapy over 10 years. Ann Allergy Asthma Immunol. 2014; 112:79–81. PMID: 24331403.15. Senna G, Ridolo E, Calderon M, Lombardi C, Canonica GW, Passalacqua G. Evidence of adherence to allergen-specific immunotherapy. Curr Opin Allergy Clin Immunol. 2009; 9:544–548. PMID: 19823080.
Article16. Anghel LA, Farcas¸ AM, Oprean RN. Medication adherence and persistence in patients with autoimmune rheumatic diseases: a narrative review. Patient Prefer Adherence. 2018; 12:1151–1166. PMID: 30013327.
Article17. Chan W, Chen A, Tiao D, Selinger C, Leong R. Medication adherence in inflammatory bowel disease. Intest Res. 2017; 15:434–445. PMID: 29142511.
Article18. Kiel MA, Röder E, Gerth van Wijk R, Al MJ, Hop WC, Rutten-van Mölken MP. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol. 2013; 132:353–360. PMID: 23651609.
Article19. Hommers L, Ellert U, Scheidt-Nave C, Langen U. Factors contributing to conductance and outcome of specific immunotherapy: data from the German National Health Interview and Examination Survey 1998. Eur J Public Health. 2007; 17:278–284. PMID: 17060335.
Article20. Donahue JG, Greineder DK, Connor-Lacke L, Canning CF, Platt R. Utilization and cost of immunotherapy for allergic asthma and rhinitis. Ann Allergy Asthma Immunol. 1999; 82:339–347. PMID: 10227332.
Article21. Boguniewicz M, Alexis AF, Beck LA, Block J, Eichenfield LF, Fonacier L, et al. Expert perspectives on management of moderate-to-severe atopic dermatitis: a multidisciplinary consensus addressing current and emerging therapies. J Allergy Clin Immunol Pract. 2017; 5:1519–1531. PMID: 28970084.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Allergen Specific Immunotherapy in Allergic Rhinitis
- Sublingual immunotherapy for allergic rhinitis
- Allergen specific IgG antibodies in nasal secretion during allergen specific immunotherapy
- Two Cases of Atopic Dermatitis Improved by Combination Treatment of Allergen-Specific Immunotherapy and Histamine-Immunoglobulin Complex
- Allergen-Specific Immunotherapies for Food Allergy