Korean J Radiol.
2019 May;20(5):781-790. 10.3348/kjr.2018.0273.
Dynamic Contrast-Enhanced Ultrasound of Gastric Cancer: Correlation with Perfusion CT and Histopathology
- Affiliations
-
- 1Department of Radiology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. shkim7071@gmail.com
- 2Institute of Radiation Medicine, Seoul National University Hospital, Seoul, Korea.
- KMID: 2442712
- DOI: http://doi.org/10.3348/kjr.2018.0273
Abstract
OBJECTIVE
To assess the relationship between contrast-enhanced ultrasound (CEUS) parameters and perfusion CT (PCT) parameters of gastric cancers and their correlation with histologic features.
MATERIALS AND METHODS
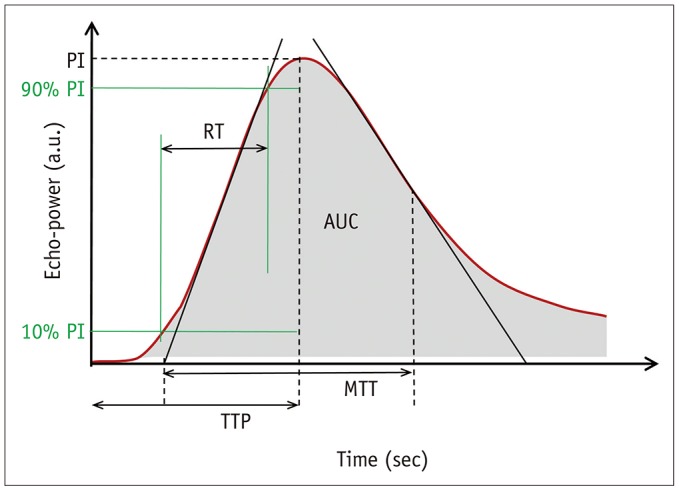
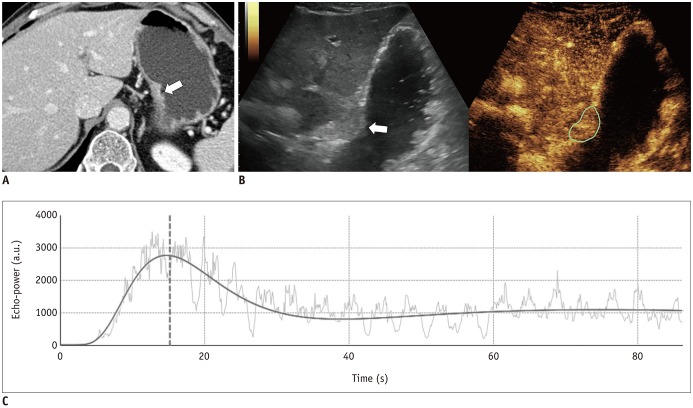
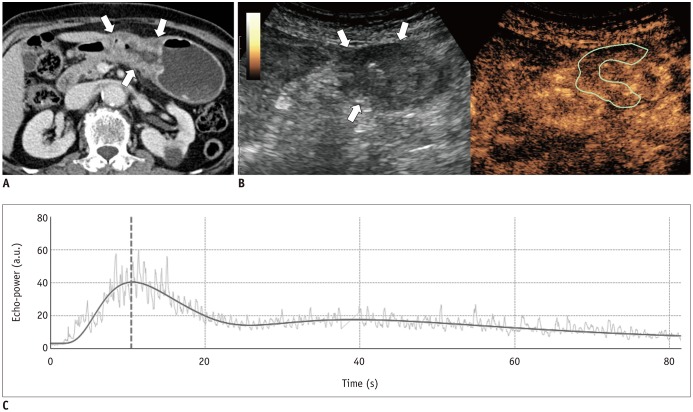
This prospective study was approved by our Institutional Review Board. We included 43 patients with pathologically-proven gastric cancers undergoing CEUS using SonoVue® (Bracco) and PCT on the same day. Correlation between the CEUS parameters (peak intensity [PI], area under the curve [AUC], rise time [RT] from 10% to 90% of PI, time to peak [TTPUS], and mean transit time [MTTUS]) and PCT parameters (blood flow, blood volume, TTPCT, MTTCT, and permeability surface product) of gastric cancers were analyzed using Spearman's rank correlation test. In cases of surgical resection, the CEUS and PCT parameters were compared according to histologic features using Mann-Whitney test.
RESULTS
CEUS studies were of diagnostic quality in 88.4% (38/43) of patients. Among the CEUS parameters of gastric cancers, RT and TTPUS showed significant positive correlations with TTPCT (rho = 0.327 and 0.374, p = 0.045 and 0.021, respectively); PI and AUC were significantly higher in well-differentiated or moderately-differentiated tumors (n = 4) than poorly-differentiated tumors (n = 18) (p = 0.026 and 0.033, respectively), whereas MTTCT showed significant differences according to histologic types (poorly cohesive carcinoma [PCC] vs. non-PCC), T-staging (≤ T2 vs. ≥ T3), N-staging (N0 vs. N-positive), and epidermal growth factor receptor expression (≤ faint vs. ≥ moderate staining) (p values < 0.05).
CONCLUSION
In patients with gastric cancers, CEUS is technically feasible for the quantification of tumor perfusion and may provide correlative and complementary information to that of PCT, which may allow prediction of histologic features.
MeSH Terms
Figure
Reference
-
1. Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012; 380:1840–1850. PMID: 23079588.
Article2. De Vita F, Giuliani F, Galizia G, Belli C, Aurilio G, Santabarbara G, et al. Neo-adjuvant and adjuvant chemotherapy of gastric cancer. Ann Oncol. 2007; 18(Suppl 6):vi120–vi123. PMID: 17591804.
Article3. Shah MA, Ajani JA. Gastric cancer--an enigmatic and heterogeneous disease. JAMA. 2010; 303:1753–1754. PMID: 20442394.
Article4. Garcia-Figueiras R, Goh VJ, Padhani AR, Baleato-Gonzalez S, Garrido M, Leon L, et al. CT perfusion in oncologic imaging: a useful tool? AJR Am J Roentgenol. 2013; 200:8–19. PMID: 23255736.5. Padhani AR. Dynamic contrast-enhanced MRI in clinical oncology: current status and future directions. J Magn Reson Imaging. 2002; 16:407–422. PMID: 12353256.
Article6. Kim SH, Kamaya A, Willmann JK. CT perfusion of the liver: principles and applications in oncology. Radiology. 2014; 272:322–344. PMID: 25058132.
Article7. O'Connor JP, Jackson A, Parker GJ, Roberts C, Jayson GC. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol. 2012; 9:167–177. PMID: 22330689.8. Frohlich E, Muller R, Cui XW, Schreiber-Dietrich D, Dietrich CF. Dynamic contrast-enhanced ultrasound for quantification of tissue perfusion. J Ultrasound Med. 2015; 34:179–196. PMID: 25614391.9. Jahng GH, Li KL, Ostergaard L, Calamante F. Perfusion magnetic resonance imaging: a comprehensive update on principles and techniques. Korean J Radiol. 2014; 15:554–577. PMID: 25246817.
Article10. Lu Q, Huang BJ, Wang WP, Li CX, Xue LY. Qualitative and quantitative analysis with contrast-enhanced ultrasonography: diagnosis value in hypoechoic renal angiomyolipoma. Korean J Radiol. 2015; 16:334–341. PMID: 25741195.
Article11. Lv WF, Han JK, Cheng DL, Zhou CZ, Ni M, Lu D. CT perfusion imaging can predict patients' survival and early response to transarterial chemo-lipiodol infusion for liver metastases from colorectal cancers. Korean J Radiol. 2015; 16:810–820. PMID: 26175580.
Article12. Zhang H, Pan Z, Du L, Yan C, Ding B, Song Q, et al. Advanced gastric cancer and perfusion imaging using a multidetector row computed tomography: correlation with prognostic determinants. Korean J Radiol. 2008; 9:119–127. PMID: 18385558.
Article13. Lee DH, Kim SH, Im SA, Oh DY, Kim TY, Han JK. Multiparametric fully-integrated 18-FDG PET/MRI of advanced gastric cancer for prediction of chemotherapy response: a preliminary study. Eur Radiol. 2016; 26:2771–2778. PMID: 26615555.
Article14. Joo I, Lee JM, Han JK, Yang HK, Lee HJ, Choi BI. Dynamic contrast-enhanced MRI of gastric cancer: correlation of the perfusion parameters with pathological prognostic factors. J Magn Reson Imaging. 2015; 41:1608–1614. PMID: 25044978.
Article15. Li T, Lu M, Song J, Wu P, Cheng X, Zhang Z. Improvement to ultrasonographical differential diagnosis of gastric lesions: the value of contrast enhanced sonography with gastric distention. PLoS One. 2017; 12:e0182332. PMID: 28783738.
Article16. Xue H, Ge HY, Miao LY, Wang SM, Zhao B, Wang JR, et al. Differential diagnosis of gastric cancer and gastritis: the role of contrast-enhanced ultrasound (CEUS). Abdom Radiol (NY). 2017; 42:802–809. PMID: 27761613.
Article17. Yan C, Bao X, Shentu W, Chen J, Liu C, Ye Q, et al. Preoperative gross classification of gastric adenocarcinoma: comparison of double contrast-enhanced ultrasound and multi-detector row CT. Ultrasound Med Biol. 2016; 42:1431–1440. PMID: 27072076.
Article18. Li S, Huang P, Wang Z, Chen J, Xu H, Wang L, et al. Preoperative T staging of advanced gastric cancer using double contrast-enhanced ultrasound. Ultraschall Med. 2012; 33:E218–E224. PMID: 22744445.
Article19. Tranquart F, Mercier L, Frinking P, Gaud E, Arditi M. Perfusion quantification in contrast-enhanced ultrasound (CEUS)--ready for research projects and routine clinical use. Ultraschall Med. 2012; 33(Suppl 1):S31–S38. PMID: 22723027.20. Shiyan L, Pintong H, Zongmin W, Fuguang H, Zhiqiang Z, Yan Y, et al. The relationship between enhanced intensity and microvessel density of gastric carcinoma using double contrast-enhanced ultrasonography. Ultrasound Med Biol. 2009; 35:1086–1091. PMID: 19419811.
Article21. Ang J, Hu L, Huang PT, Wu JX, Huang LN, Cao CH, et al. Contrast-enhanced ultrasonography assessment of gastric cancer response to neoadjuvant chemotherapy. World J Gastroenterol. 2012; 18:7026–7032. PMID: 23323004.
Article22. Ranganath PG, Robbin ML, Back SJ, Grant EG, Fetzer DT. Practical advantages of contrast-enhanced ultrasound in abdominopelvic radiology. Abdom Radiol (NY). 2018; 43:998–1012. PMID: 29332247.
Article23. Lee DH, Kim SH, Joo I, Han JK. CT Perfusion evaluation of gastric cancer: correlation with histologic type. Eur Radiol. 2018; 28:487–495. PMID: 28779403.
Article24. Jang JY, Kim MY, Jeong SW, Kim TY, Kim SU, Lee SH, et al. Current consensus and guidelines of contrast enhanced ultrasound for the characterization of focal liver lesions. Clin Mol Hepatol. 2013; 19:1–16. PMID: 23593604.
Article25. Zongqiong S, Xiaohong L, Wei C, Jiangfeng Z, Yuxi G, Zhihui X, et al. CT perfusion imaging of the stomach: a quantitative analysis according to different degrees of adenocarcinoma cell differentiation. Clin Imaging. 2016; 40:558–562. PMID: 27133704.
Article26. Lang SA, Moser C, Gehmert S, Pfister K, Hackl C, Schnitzbauer AA, et al. Contrast-enhanced ultrasound (CEUS) detects effects of vascular disrupting therapy in an experimental model of gastric cancer. Clin Hemorheol Microcirc. 2014; 56:287–299. PMID: 23271198.
Article27. Goetti R, Reiner CS, Knuth A, Klotz E, Stenner F, Samaras P, et al. Quantitative perfusion analysis of malignant liver tumors: dynamic computed tomography and contrast-enhanced ultrasound. Invest Radiol. 2012; 47:18–24. PMID: 21788906.28. Meijerink MR, van Waesberghe JH, van Schaik C, Boven E, van der Veldt AA, van den Tol P, et al. Perfusion CT and US of colorectal cancer liver metastases: a correlative study of two dynamic imaging modalities. Ultrasound Med Biol. 2010; 36:1626–1636. PMID: 20800954.
Article29. Zhou JH, Zheng W, Cao LH, Liu M, Luo RZ, Han F, et al. Quantitative evaluation of viable tissue perfusion changes with contrast-enhanced greyscale ultrasound in a mouse hepatoma model following treatment with different doses of thalidomide. Br J Radiol. 2011; 84:826–832. PMID: 21224299.
Article30. Zhuang H, Yang ZG, Chen HJ, Peng YL, Li L. Time-intensity curve parameters in colorectal tumours measured using double contrast-enhanced ultrasound: correlations with tumour angiogenesis. Colorectal Dis. 2012; 14:181–187. PMID: 21689263.
Article31. Wang J, Lv F, Fei X, Cui Q, Wang L, Gao X, et al. Study on the characteristics of contrast-enhanced ultrasound and its utility in assessing the microvessel density in ovarian tumors or tumor-like lesions. Int J Biol Sci. 2011; 7:600–606. PMID: 21614152.
Article32. Yang JC, Tang J, Li Y, Fei X, Shi H. Contrast-enhanced transrectal ultrasound for assessing vascularization of hypoechoic BPH nodules in the transition and peripheral zones: comparison with pathological examination. Ultrasound Med Biol. 2008; 34:1758–1764. PMID: 18524461.
Article33. Kawamura M, Naganuma H, Shibuya R, Kikuchi T, Sakai Y, Nagasaki F, et al. Analysis of microvascular density in early gastric carcinoma using magnifying endoscopy with narrow-band imaging. Endosc Int Open. 2016; 4:E832–E837. PMID: 27540569.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatocellular Carcinoma Detected on Liver Ultrasound but not Confirmed on Dynamic Contrast-enhanced CT
- Primary Lung Cancer: Utility of Contrast-enhanced Dynamic CT in Diagnosis with Histopathologic Correlation
- Enhancement Patterns of Hepatic Metastasis from Stomach Cancer at Multi-phase Incremental Bolus Dynamic CT
- Contrast Enhanced US in the Abdomen
- Contrast-enhanced ultrasound of hepatocellular carcinoma: where do we stand?