J Korean Soc Radiol.
2019 Mar;80(2):283-293. 10.3348/jksr.2019.80.2.283.
Superficially Palpable Masses of the Scalp and Face: A Pictorial Essay
- Affiliations
-
- 1Department of Radiology, Nowon Eulji Medical Center, Eulji University School of Medicine, Seoul, Korea. jkan0831@eulji.ac.kr
- KMID: 2442519
- DOI: http://doi.org/10.3348/jksr.2019.80.2.283
Abstract
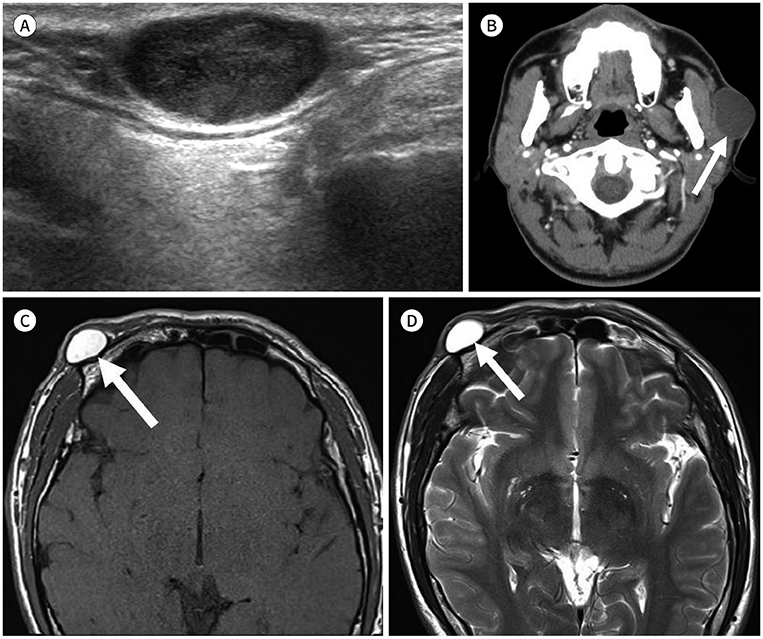
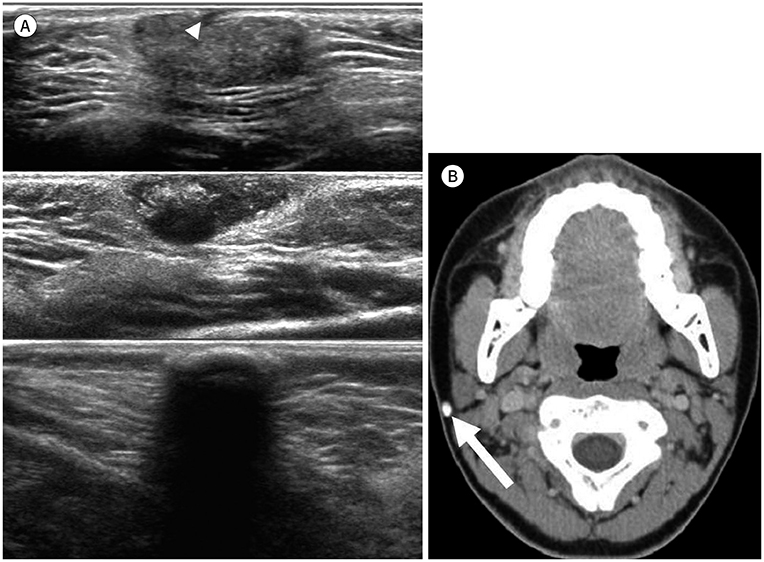
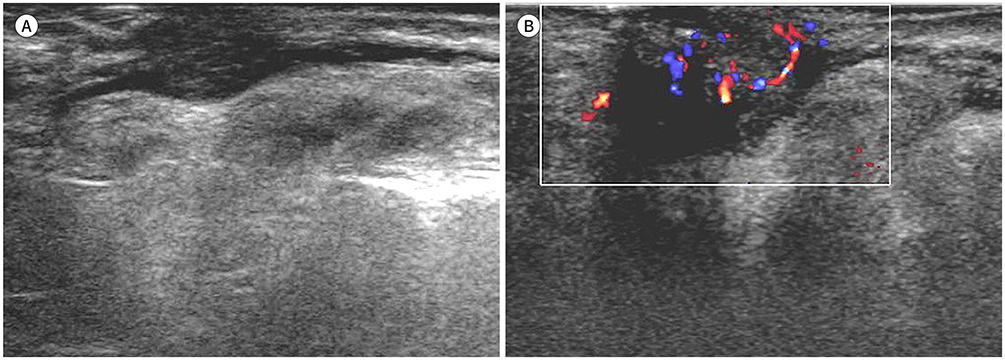
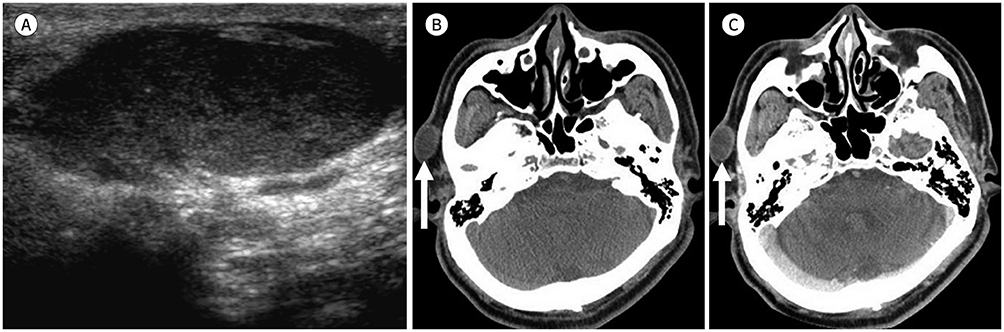
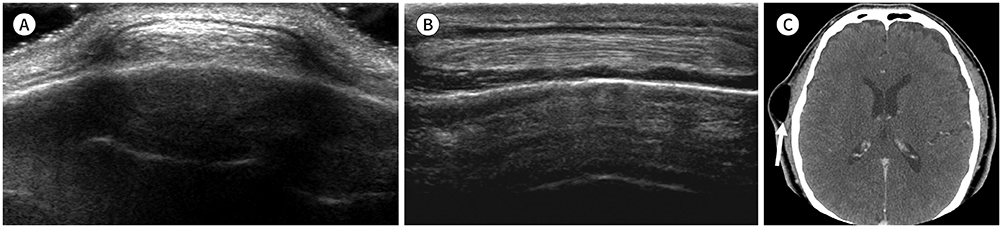
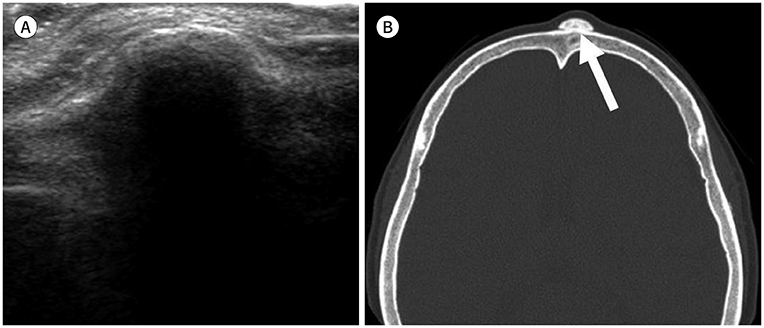
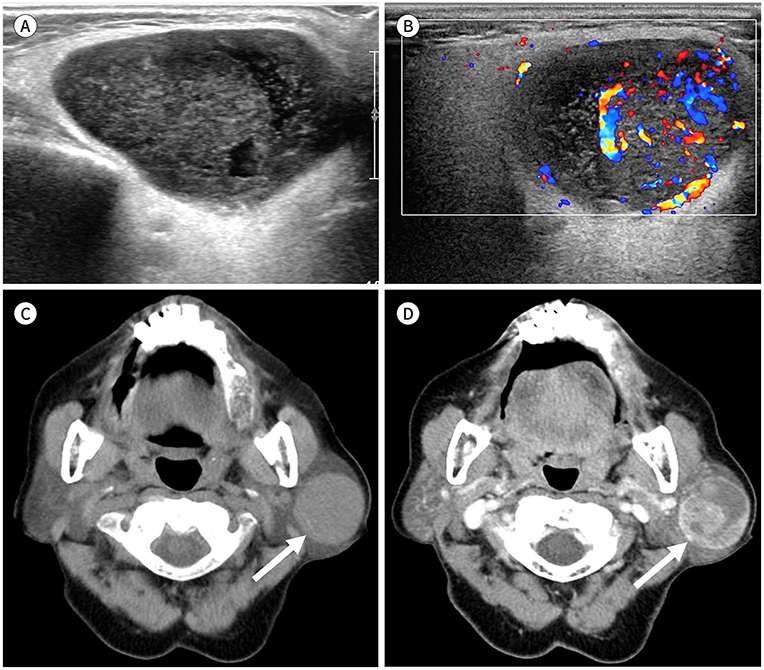
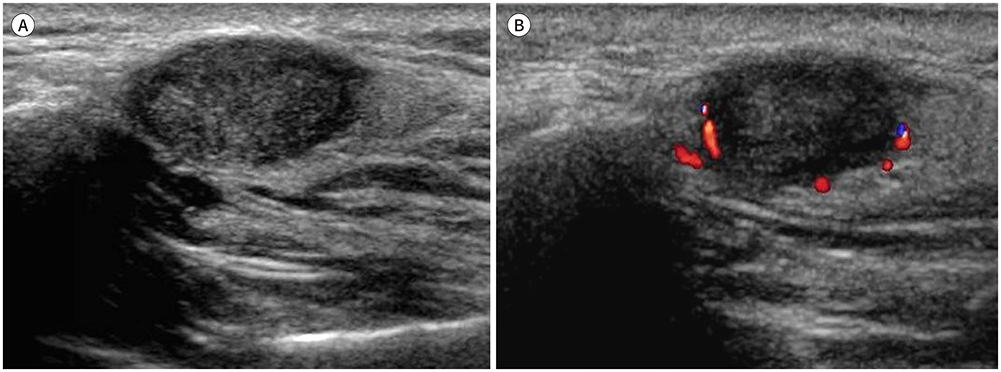
- Palpable lesions of the scalp and face are common in clinical practice. They are usually small and benign, and the lesions tend to be treated simply according to the clinical symptoms. However, radiologic evaluation is often performed to determine the exact type and location of a lesion to ensure appropriate management. Ultrasonography is useful as a primary and definitive modality for evaluating small superficial lesions. CT and MRI are better for characterizing soft tissue features and provide superior soft tissue resolution. This article discusses various lesions and their imaging findings of the scalp and face that may present as superficially palpable masses.
Figure
Reference
-
1. El-Naggar AK, Chan JK, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours. 4th ed. Lyon: IARC press;2017.2. Eskey CJ, Robson CD, Weber AL. Imaging of benign and malignant soft tissue tumors of the neck. Radiol Clin North Am. 2000; 38:1091–1104.
Article3. Beaman FD, Kransdorf MJ, Andrews TR, Murphey MD, Arcara LK, Keeling JH. Superficial soft-tissue masses: analysis, diagnosis, and differential considerations. Radiographics. 2007; 27:509–523.
Article4. Huang CC, Ko SF, Huang HY, Ng SH, Lee TY, Lee YW, et al. Epidermal cysts in the superficial soft tissue: sonographic features with an emphasis on the pseudotestis pattern. J Ultrasound Med. 2011; 30:11–17.5. Nigam JS, Bharti JN, Nair V, Gargade CB, Deshpande AH, Dey B, et al. Epidermal cysts: a clinicopathological analysis with emphasis on unusual findings. Int J Trichology. 2017; 9:108–112.6. Taira N, Aogi K, Ohsumi S, Takashima S, Kawamura S, Nishimura R. Epidermal inclusion cyst of the breast. Breast Cancer. 2007; 14:434–437.
Article7. Fisher AR, Mason PH, Wagenhals KS. Ruptured plantar epidermal inclusion cyst. AJR Am J Roentgenol. 1998; 171:1709–1710.
Article8. Hong SH, Chung HW, Choi JY, Koh YH, Choi JA, Kang HS. MRI findings of subcutaneous epidermal cysts: emphasis on the presence of rupture. AJR Am J Roentgenol. 2006; 186:961–966.
Article9. Stone MS. Cysts. In : Bolognia JL, Schaffer JV, Cerroni L, editors. Dermatology. 4th ed. Philadelphia: Elsevier;2018. p. 1917–1929.10. Jin W, Ryu KN, Kim GY, Kim HC, Lee JH, Park JS. Sonographic findings of ruptured epidermal inclusion cysts in superficial soft tissue: emphasis on shapes, pericystic changes, and pericystic vascularity. J Ultrasound Med. 2008; 27:171–176.11. Yasumoto M, Shibuya H, Gomi N, Kasuga T. Ultrasonographic appearance of dermoid and epidermoid cysts in the head and neck. J Clin Ultrasound. 1991; 19:455–461.
Article12. Yuan WH, Hsu HC, Lai YC, Chou YH, Li AF. Differences in sonographic features of ruptured and unruptured epidermal cysts. J Ultrasound Med. 2012; 31:265–272.
Article13. Kim HK, Kim SM, Lee SH, Racadio JM, Shin MJ. Subcutaneous epidermal inclusion cysts: ultrasound (US) and MR imaging findings. Skeletal Radiol. 2011; 40:1415–1419.
Article14. Langer JE, Ramchandani P, Siegelman ES, Banner MP. Epidermoid cysts of the testicle: sonographic and MR imaging features. AJR Am J Roentgenol. 1999; 173:1295–1299.
Article15. Shibata T, Hatori M, Satoh T, Ehara S, Kokubun S. Magnetic resonance imaging features of epidermoid cyst in the extremities. Arch Orthop Trauma Surg. 2003; 123:239–241.
Article16. Alsaad KO, Obaidat NA, Ghazarian D. Skin adnexal neoplasms-part 1: an approach to tumours of the pilosebaceous unit. J Clin Pathol. 2007; 60:129–144.
Article17. Haller JO, Kassner EG, Ostrowitz A, Kottmeler PK, Perfschuk LP. Pilomatrixoma (calcifying epithelioma of Malherbe): radiographic features. Radiology. 1977; 123:151–153.
Article18. Garioni E, Danesino GM, Madonia L. Pilomatricoma: sonographic features. J Ultrasound. 2008; 11:76–78.
Article19. Lin SF, Xu SH, Xie ZL. Calcifying epithelioma of malherbe (Pilomatrixoma): clinical and sonographic features. J Clin Ultrasound. 2018; 46:3–7.
Article20. Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, et al. Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015; 136:e203–e214.
Article21. George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014; 18:S117–S120.
Article22. Buckmiller LM, Richter GT, Suen JY. Diagnosis and management of hemangiomas and vascular malformations of the head and neck. Oral Dis. 2010; 16:405–418.
Article23. Griauzde J, Srinivasan A. Imaging of vascular lesions of the head and neck. Radiol Clin North Am. 2015; 53:197–213.
Article24. Baer AH, Parmar HA, DiPietro MA, Kasten SJ, Mukherji SK. Hemangiomas and vascular malformations of the head and neck: a simplified approach. Neuroimaging Clin N Am. 2011; 21:641–658.
Article25. Mittal MK, Malik A, Sureka B, Thukral BB. Cystic masses of neck: a pictorial review. Indian J Radiol Imaging. 2012; 22:334–343.
Article26. Adams A, Mankad K, Offiah C, Childs L. Branchial cleft anomalies: a pictorial review of embryological development and spectrum of imaging findings. Insights Imaging. 2016; 7:69–76.
Article27. Brown RE, Harave S. Diagnostic imaging of benign and malignant neck masses in children-a pictorial review. Quant Imaging Med Surg. 2016; 6:591–604.
Article28. Cappabianca S, Colella G, Pezzullo MG, Russo A, Iaselli F, Brunese L, et al. Lipomatous lesions of the head and neck region: imaging findings in comparison with histological type. Radiol Med. 2008; 113:758–770.
Article29. Bancroft LW, Kransdorf MJ, Peterson JJ, O’Connor MI. Benign fatty tumors: classification, clinical course, imaging appearance, and treatment. Skeletal Radiol. 2006; 35:719–733.
Article30. Lloret I, Server A, Taksdal I. Calvarial lesions: a radiological approach to diagnosis. Acta Radiol. 2009; 50:531–542.
Article31. Colas L, Caron S, Cotten A. Skull vault lesions: a review. AJR Am J Roentgenol. 2015; 205:840–847.
Article32. Garfinkle J, Melançon D, Cortes M, Tampieri D. Imaging pattern of calvarial lesions in adults. Skeletal Radiol. 2011; 40:1261–1273.
Article33. Yalçin O, Yildirim T, Kizilkiliç O, Hürcan CE, Koç Z, Aydin V, et al. CT and MRI findings in calvarial non-infectious lesions. Diagn Interv Radiol. 2007; 13:68–74.34. Thoeny HC. Imaging of salivary gland tumours. Cancer Imaging. 2007; 7:52–62.
Article35. Lee YY, Wong KT, King AD, Ahuja AT. Imaging of salivary gland tumours. Eur J Radiol. 2008; 66:419–436.
Article36. Cantisani V, David E, Sidhu PS, Sacconi B, Greco A, Pandolfi F, et al. Parotid gland lesions: multiparametric ultrasound and MRI features. Ultraschall Med. 2016; 37:454–471.
Article37. Barton F, Branstetter IV. Mucoepidermoid carcinoma, parotid. In : Harnsberger HR, Glastonbury CM, Michel MA, Koch BL, editors. Diagnostic imaging-head and neck. Salt Lake: Amirsys Inc;2005.38. Som PM, Brandwein MS. Salivary glands: anatomy and pathology. In : Som PM, Curtin DC, editors. Head and neck imaging. 4th ed. St. Louis: Mosby;2003. p. 2005–2133.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sonographic Features of Palpable Breast and Axillary Lesions in Adult Male Patients: A Pictorial Essay
- Multi-Detector CT Findings of Palpable Chest Wall Masses in Children: A Pictorial Essay
- Imaging Features of the Mesenchymal Tumors of the Breast according to WHO Classification: A Pictorial Essay
- Postoperative Imaging Findings of Colorectal Surgery: A Pictorial Essay
- Spectrum of Axillary Disorders (Excluding Metastasis from Breast Cancer): Radiological and Pathological Correlation: A Pictorial Essay