J Breast Cancer.
2019 Mar;22(1):29-37. 10.4048/jbc.2019.22.e15.
Circulating Plasmacytoid and Myeloid Dendritic Cells in Breast Cancer Patients: A Pilot Study
- Affiliations
-
- 1Department of Surgery, Ewha Womans University College of Medicine, Seoul, Korea. mbit@ewha.ac.kr
- 2Department of Surgery, Chungnam National University College of Medicine, Daejeon, Korea.
- KMID: 2441849
- DOI: http://doi.org/10.4048/jbc.2019.22.e15
Abstract
- PURPOSE
Dendritic cells (DC) are a class of bone marrow-derived cells found in the blood, epithelia, and lymphoid tissues, and are the most efficient antigen presenting cells. The number and function of DC can change dramatically in cancer patients. The aim of this study is to correlate the levels of circulating DC subsets with clinical characteristics in breast cancer patients.
METHODS
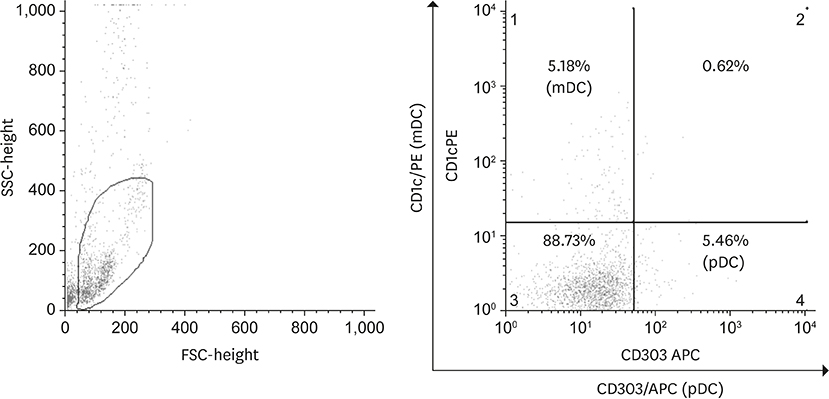
Peripheral blood samples were collected from 53 untreated breast cancer patients before surgery between January 2013 and November 2013. Forty-one healthy, age-matched volunteers served as the control group. The phenotypes of circulating plasmacytoid DCs (pDCs) and myeloid DCs (mDCs) were determined using fluorescence activated cell sorting assays. Correlations between DCs immunophenotypes and clinicopathologic characteristics of these breast cancer patients were then determined.
RESULTS
Patients with breast cancer had higher levels of pDCs (p = 0.046). No relationships were observed with tumor stage and intrinsic subtype. Estrogen receptor (ER) positive patients had higher levels of mDCs than ER negative patients (p = 0.025) and human epidermal growth factor receptor 2 (HER-2) positive patients had higher levels of pDCs than HER-2 (p = 0.040). No relationships were observed with T stage, N stage, Ki67 index, histologic grade, nuclear grade, and lymphovascular invasion. In multiple regression analysis, patients with HER-2 positive breast cancer had higher levels of pDCs than HER-2 negative patients (p = 0.026).
CONCLUSION
An increase of pDCs in the peripheral blood of breast cancer patients was observed and patients with HER-2 positive breast cancer had higher levels of circulating pDCs than did HER-2 negative patients. Our results suggest that expression of DCs can differ according to breast cancer subtype and indicate that, with further investigation, DC expression has the possibility of being presented as a prognostic factor.
Keyword
MeSH Terms
Figure
Reference
-
1. Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology. 2013; 140:22–30.
Article2. Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014; 14:392–404.
Article3. Reynolds G, Haniffa M. Human and mouse mononuclear phagocyte networks: a tale of two species? Front Immunol. 2015; 6:330.
Article4. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998; 392:245–252.
Article5. Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997; 90:3245–3287.
Article6. Steinman RM. Linking innate to adaptive immunity through dendritic cells. Novartis Found Symp. 2006; 279:101–109.
Article7. Liu Y, Cao X. Intratumoral dendritic cells in the anti-tumor immune response. Cell Mol Immunol. 2015; 12:387–390.
Article8. Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004; 10:7466–7474.
Article9. Mego M, Gao H, Cohen EN, Anfossi S, Giordano A, Tin S, et al. Circulating tumor cells (CTCs) are associated with abnormalities in peripheral blood dendritic cells in patients with inflammatory breast cancer. Oncotarget. 2017; 8:35656–35668.
Article10. Ban YL, Kong BH, Qu X, Yang QF, Ma YY. BDCA-1+, BDCA-2+ and BDCA-3+ dendritic cells in early human pregnancy decidua. Clin Exp Immunol. 2008; 151:399–406.
Article11. Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001; 166:678–689.
Article12. Hart DN, Hill GR. Dendritic cell immunotherapy for cancer: application to low-grade lymphoma and multiple myeloma. Immunol Cell Biol. 1999; 77:451–459.
Article13. Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res. 1997; 3:483–490.14. Kini Bailur J, Gueckel B, Pawelec G. Prognostic impact of high levels of circulating plasmacytoid dendritic cells in breast cancer. J Transl Med. 2016; 14:151.
Article15. Wojas K, Tabarkiewicz J, Jankiewicz M, Roliński J. Dendritic cells in peripheral blood of patients with breast and lung cancer--a pilot study. Folia Histochem Cytobiol. 2004; 42:45–48.16. Pinto A, Rega A, Crother TR, Sorrentino R. Plasmacytoid dendritic cells and their therapeutic activity in cancer. OncoImmunology. 2012; 1:726–734.
Article17. Piccioli D, Sammicheli C, Tavarini S, Nuti S, Frigimelica E, Manetti AG, et al. Human plasmacytoid dendritic cells are unresponsive to bacterial stimulation and require a novel type of cooperation with myeloid dendritic cells for maturation. Blood. 2009; 113:4232–4239.
Article18. Lou Y, Liu C, Kim GJ, Liu YJ, Hwu P, Wang G. Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses. J Immunol. 2007; 178:1534–1541.
Article19. Chevolet I, Speeckaert R, Schreuer M, Neyns B, Krysko O, Bachert C, et al. Clinical significance of plasmacytoid dendritic cells and myeloid-derived suppressor cells in melanoma. J Transl Med. 2015; 13:9.
Article20. Sisirak V, Faget J, Vey N, Blay JY, Ménétrier-Caux C, Caux C, et al. Plasmacytoid dendritic cells deficient in IFNα production promote the amplification of FOXP3+ regulatory T cells and are associated with poor prognosis in breast cancer patients. OncoImmunology. 2013; 2:e22338.21. Milani A, Sangiolo D, Montemurro F, Aglietta M, Valabrega G. Active immunotherapy in HER2 overexpressing breast cancer: current status and future perspectives. Ann Oncol. 2013; 24:1740–1748.
Article22. Lowenfeld L, Mick R, Datta J, Xu S, Fitzpatrick E, Fisher CS, et al. Dendritic cell vaccination enhances immune responses and induces regression of HER2pos DCIS independent of route: results of randomized selection design trial. Clin Cancer Res. 2017; 23:2961–2971.
Article23. Bailur JK, Gueckel B, Derhovanessian E, Pawelec G. Presence of circulating HER2-reactive CD8 + T-cells is associated with lower frequencies of myeloid-derived suppressor cells and regulatory T cells, and better survival in older breast cancer patients. Breast Cancer Res. 2015; 17:34.
Article24. Schrøder M, Melum GR, Landsverk OJ, Bujko A, Yaqub S, Gran E, et al. CD1c-expression by monocytes - implications for the use of commercial CD1c+ dendritic cell isolation kits. PLoS One. 2016; 11:e0157387.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Woman with Blastic Plasmacytoid Dendritic Cell Neoplasm
- Plasmacytoid dendritic cell neoplasms
- The Role of Plasmacytoid and Myeloid Dendritic Cells in Induction of Asthma in a Mouse Model and the Effect of a TLR9 Agonist on Dendritic Cells
- A Case of Blastic Plasmacytoid Dendritic Cell Neoplasm in Child
- Helper T Cell Polarizing Through Dendritic Cells