Allergy Asthma Immunol Res.
2018 Nov;10(6):675-685. 10.4168/aair.2018.10.6.675.
Efficacy and Safety of Sublingual Immunotherapy in Elderly Rhinitis Patients Sensitized to House Dust Mites
- Affiliations
-
- 1Division of Respiratory, Allergy and Critical Care Medicine, Konyang University College of Medicine, Daejeon, Korea.
- 2Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. hspark@ajou.ac.kr
- 3Division of Allergy and Immunology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Division of Respiratory and Critical Care Medicine, Korea University College of Medicine, Seoul, Korea.
- 5Division of Pulmonary, Allergy, and Critical Care Medicine, Hallylm University Sacred Heart Hospital, Anyang, Korea.
- 6Department of Statistics, Clinical Trial Center, Ajou University Medical Center, Suwon, Korea.
- KMID: 2441816
- DOI: http://doi.org/10.4168/aair.2018.10.6.675
Abstract
- PURPOSE
This study aims to determine the efficacy and safety of house dust mite (HDM)-sublingual immunotherapy (SLIT) in elderly patients with AR.
METHODS
A total of 45 patients aged ≥ 60 years with HDM-induced AR who had ≥ 3 A/H ratio on skin prick test and/or ≥ 0.35 IU/L to both Dermatophagoides farinae and Dermatophagoides pteronyssinus by ImmunoCAP were enrolled in 4 university hospitals. To evaluate additional effects of HDM-SLIT, they were randomized to the SLIT-treated group (n = 30) or control group (n = 15). Rhinoconjunctivitis total symptom score (RTSS), rhinoscopy score, Korean rhinoconjunctivitis quality of life questionnaire, rhinitis control assessment test, asthma control test scores, and adverse reactions, were assessed at the first visit (V1) and after 1 year of treatment (V5); for immunological evaluation, serum levels of HDM-specific immunoglobulin A/IgE/IgG1/IgG4 antibodies and basophil response to HDMs were compared between V1 and V5 in both groups.
RESULTS
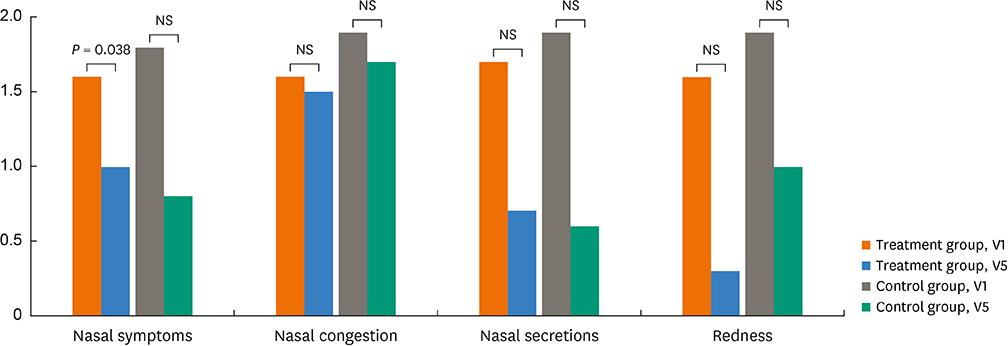
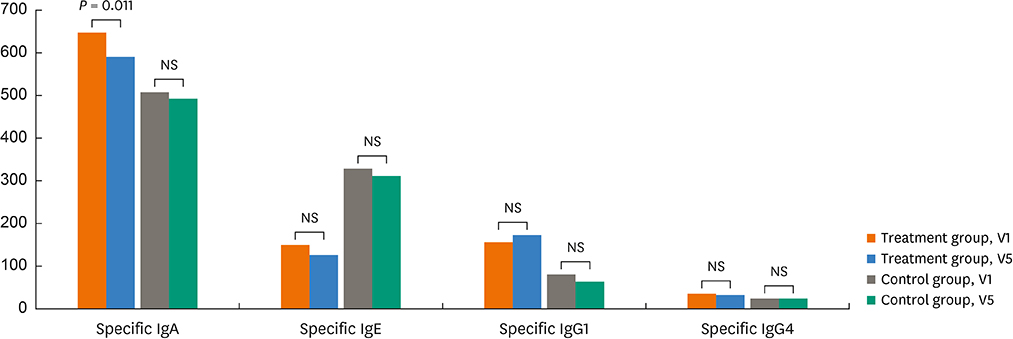
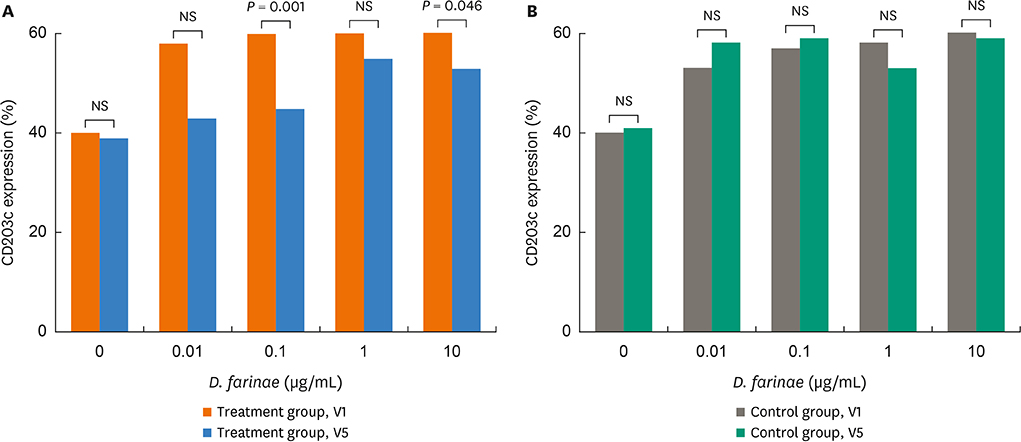
There were no significant differences in demographics, RTSS, skin reactivity to HDMs, or serum total/specific IgE levels to HDMs (P < 0.05, respectively) between the 2 groups. Nasal symptom score and RTSS decreased significantly at year 1 in the 2 groups (P < 0.05). There were no significant differences in percent decrease in nasal symptom score and RTSS at year 1 between the 2 groups (P < 0.05); however, rhinoscopic nasal symptom score decreased significantly in the SLIT-treated group (P < 0.05). Immunological studies showed that serum specific IgA levels (not specific IgE/IgG) and CD203c expression on basophils decreased significantly at V5 in the SLIT-treated group (P = 0.011 and P = 0.001, respectively), not in the control group. The control group required more medications compared to the treatment group, but there were no differences in adverse reactions.
CONCLUSIONS
It is suggested that HDM-SLIT for 1 year could induce symptom improvement and may induce immunomodulation in elderly rhinitis patients.
MeSH Terms
-
Aged*
Antibodies
Asthma
Basophils
Demography
Dermatophagoides farinae
Dermatophagoides pteronyssinus
Dust*
Hospitals, University
Humans
Immunoglobulin A
Immunoglobulin E
Immunoglobulins
Immunomodulation
Immunotherapy
Pyroglyphidae*
Quality of Life
Rhinitis*
Rhinitis, Allergic
Skin
Sublingual Immunotherapy*
Antibodies
Dust
Immunoglobulin A
Immunoglobulin E
Immunoglobulins
Figure
Reference
-
1. Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O'Donnell M, Sullivan R, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015; 385:549–562.
Article2. Slavin RG. Special considerations in treatment of allergic rhinitis in the elderly: role of intranasal corticosteroids. Allergy Asthma Proc. 2010; 31:179–184.
Article3. Scichilone N, Ventura MT, Bonini M, Braido F, Bucca C, Caminati M, et al. Choosing wisely: practical considerations on treatment efficacy and safety of asthma in the elderly. Clin Mol Allergy. 2015; 13:7.
Article4. Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013; 131:1288–96.e3.5. Bozek A, Kolodziejczyk K, Warkocka-Szoltysek B, Jarzab J. Grass pollen sublingual immunotherapy: a double-blind, placebo-controlled study in elderly patients with seasonal allergic rhinitis. Am J Rhinol Allergy. 2014; 28:423–427.
Article6. Hur GY, Lee JH, Park HS. Allergen immunotherapy for the treatment of respiratory allergies in the elderly. Curr Opin Allergy Clin Immunol. 2017; 17:304–308.
Article7. Kim MA, Ye YM, Ban GY, Shin YS, Nahm DH, Park HS. Linguistic adaptation of the rhinitis control assessment test in Korean. Allergy Asthma Respir Dis. 2017; 5:205–210.
Article8. Kwon HS, Lee SH, Yang MS, Lee SM, Kim SH, Kim DI, et al. Correlation between the Korean version of Asthma Control Test and health-related quality of life in adult asthmatics. J Korean Med Sci. 2008; 23:621–627.
Article9. Park KH, Cho JS, Lee KH, Shin SY, Moon JH, Cha CI. Rhinoconjunctivitis quality of life questionnaire (RQLQ) as an evaluator of perennial allergic rhinitis patients-the first report. Korean J Otolaryngol-Head Neck Surg. 2002; 45:254–262.10. Kim JH, Yoon MG, Seo DH, Kim BS, Ban GY, Ye YM, et al. Detection of allergen specific antibodies from nasal secretion of allergic rhinitis patients. Allergy Asthma Immunol Res. 2016; 8:329–337.
Article11. Oh JH, Hur GY, Ye YM, Kim JE, Park K, Park HS. Correlation between specific IgA and eosinophil numbers in the lavage fluid of patients with perennial allergic rhinitis. Allergy Asthma Proc. 2008; 29:152–160.
Article12. Kim JH, Kim SH, Palikhe S, Yang EM, Ye YM, Park HS. Increased basophil activation in adult patients with anaphylaxis. Ann Allergy Asthma Immunol. 2015; 115:523–5.e1.
Article13. Kim SY, Kim JH, Jang YS, Choi JH, Park S, Hwang YI, et al. The Basophil activation test is safe and useful for confirming drug-induced anaphylaxis. Allergy Asthma Immunol Res. 2016; 8:541–544.
Article14. Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016; 16:133.
Article15. Becerril Angeles M, Vázquez Merino CL, Angeles Garay U, Alvarado Moctezuma LE, Vilchis Guízar E. Prevalence of allergic diseases in the elderly. Rev Alerg Mex. 2008; 55:85–91.16. Bozek A, Ignasiak B, Filipowska B, Jarzab J. House dust mite sublingual immunotherapy: a double-blind, placebo-controlled study in elderly patients with allergic rhinitis. Clin Exp Allergy. 2013; 43:242–248.
Article17. Ohn J, Paik SH, Doh EJ, Park HS, Yoon HS, Cho S. Allergen sensitization pattern by sex: a cluster analysis in Korea. Ann Dermatol. 2017; 29:735–741.
Article18. Feng B, Xiang H, Jin H, Gao J, Huang S, Shi Y, et al. Efficacy of sublingual immunotherapy for house dust mite-induced allergic rhinitis: a meta-analysis of randomized controlled trials. Allergy Asthma Immunol Res. 2017; 9:220–228.
Article19. Wardzyńska A, Kubsik B, Kowalski ML. Comorbidities in elderly patients with asthma: association with control of the disease and concomitant treatment. Geriatr Gerontol Int. 2015; 15:902–909.
Article20. Baptist AP, Nyenhuis S. Rhinitis in the elderly. Immunol Allergy Clin North Am. 2016; 36:343–357.
Article21. Pinto JM, Jeswani S. Rhinitis in the geriatric population. Allergy Asthma Clin Immunol. 2010; 6:10.
Article22. Lue KH, Lin YH, Sun HL, Lu KH, Hsieh JC, Chou MC. Clinical and immunologic effects of sublingual immunotherapy in asthmatic children sensitized to mites: a double-blind, randomized, placebo-controlled study. Pediatr Allergy Immunol. 2006; 17:408–415.
Article23. Trebuchon F, Lhéritier-Barrand M, David M, Demoly P. Characteristics and management of sublingual allergen immunotherapy in children with allergic rhinitis and asthma induced by house dust mite allergens. Clin Transl Allergy. 2014; 4:15.
Article24. Bozek A. Pharmacological management of allergic rhinitis in the elderly. Drugs Aging. 2017; 34:21–28.
Article25. Mathur SK. Allergy and asthma in the elderly. Semin Respir Crit Care Med. 2010; 31:587–595.
Article26. Böttcher MF, Häggström P, Björkstén B, Jenmalm MC. Total and allergen-specific immunoglobulin A levels in saliva in relation to the development of allergy in infants up to 2 years of age. Clin Exp Allergy. 2002; 32:1293–1298.27. Kulis M, Saba K, Kim EH, Bird JA, Kamilaris N, Vickery BP, et al. Increased peanut-specific IgA in saliva correlates with food challenge outcomes following peanut sublingual immunotherapy. J Allergy Clin Immunol. 2012; 129:1159–1162.28. Sugimoto M, Kamemura N, Nagao M, Irahara M, Kagami S, Fujisawa T, et al. Differential response in allergen-specific IgE, IgGs, and IgA levels for predicting outcome of oral immunotherapy. Pediatr Allergy Immunol. 2016; 27:276–282.
Article29. Gómez E, Campo P, Rondón C, Barrionuevo E, Blanca-López N, Torres MJ, et al. Role of the basophil activation test in the diagnosis of local allergic rhinitis. J Allergy Clin Immunol. 2013; 132:975–976.e1-5.
Article30. Sanz ML, Sánchez G, Gamboa PM, Vila L, Uasuf C, Chazot M, et al. Allergen-induced basophil activation: CD63 cell expression detected by flow cytometry in patients allergic to Dermatophagoides pteronyssinus and Lolium perenne . Clin Exp Allergy. 2001; 31:1007–1013.31. Eberlein B, Santos AF, Mayorga C, Nopp A, Ferrer M, Rouzaire P, et al. Basophil activation testing in diagnosis and monitoring of allergic disease – an overview. Allergo J. 2016; 25:26–33.
Article32. Ebo DG, Hagendorens MM, Schuerwegh AJ, Beirens LM, Bridts CH, De Clerck LS, et al. Flow-assisted quantification of in vitro activated basophils in the diagnosis of wasp venom allergy and follow-up of wasp venom immunotherapy. Cytometry B Clin Cytom. 2007; 72:196–203.
Article33. Van Overtvelt L, Baron-Bodo V, Horiot S, Moussu H, Ricarte C, Horak F, et al. Changes in basophil activation during grass-pollen sublingual immunotherapy do not correlate with clinical efficacy. Allergy. 2011; 66:1530–1537.
Article34. Jutel M, Kosowska A, Smolinska S. Allergen immunotherapy: past, present, and future. Allergy Asthma Immunol Res. 2016; 8:191–197.
Article35. Pagano F. Therapeutic compliance in elderly patients with COPD. J Gerontol Geriat. 2016; 64:147–151.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Update of Sublingual Immunotherapy for Allergic Rhinitis
- Outcome of Sublingual Immunotherapy in Patients With Allergic Rhinitis Sensitive to House Dust Mites
- Three-Year Follow-up Results of Sublingual Immunotherapy in Patients With Allergic Rhinitis Sensitized to House Dust Mites
- Distribution of House Dust Mites in the Bedroom of Patients with Allergic Rhinitis in Pusan Area
- Sublingual Immunotherapy in Asian Children: 2-Year Follow-Up Results