Endocrinol Metab.
2019 Mar;34(1):70-79. 10.3803/EnM.2019.34.1.70.
Retrospective Analysis of the Efficacy of Dapagliflozin in Patients with Type 2 Diabetes in a Primary Clinic in Korea
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. hongsiri@hanmail.net
- 2Huh's Diabetes Center and 21st Century Diabetes and Vascular Research Institute, Seoul, Korea. huh7181827@hanmail.net
- KMID: 2441681
- DOI: http://doi.org/10.3803/EnM.2019.34.1.70
Abstract
- BACKGROUND
We aimed to retrospectively analyze the efficacy of 10 mg dapagliflozin (DAPA), which is a sodium-glucose cotransporter-2 inhibitor, in Korean patients with type 2 diabetes who visited a primary diabetes clinic.
METHODS
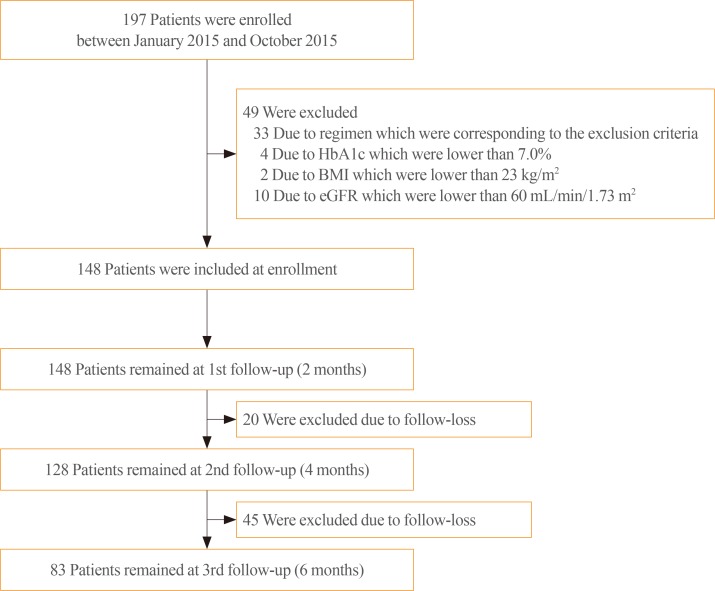
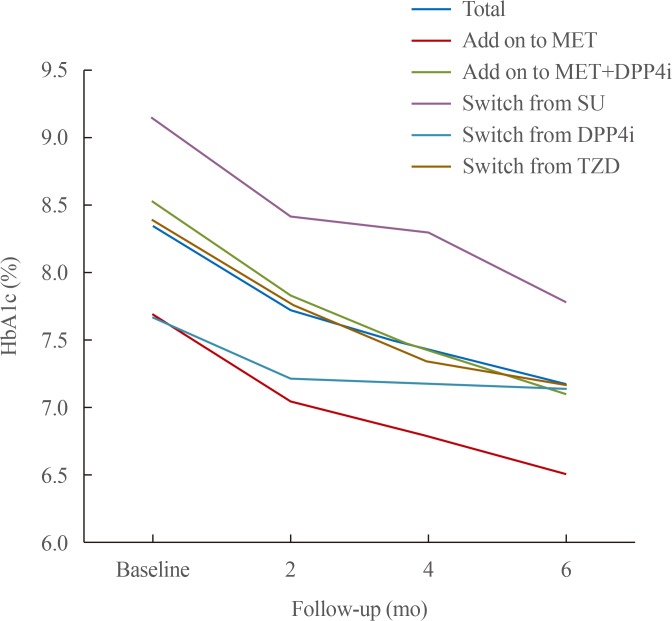
In total, 83 patients with type 2 diabetes, who received treatment with DAPA for the first time in a primary diabetes clinic between January 2015 and October 2015, were included in the study. The effect of DAPA in lowering glycosylated hemoglobin (HbA1c) levels was evaluated via chart review at 6 months follow-up. The patients were categorized into five groups according to add-on to or switched from other glucose-lowering agents: add-on to metformin (MET, n=10), add-on to MET+dipeptidyl peptidase 4 inhibitor (DPP4i, n=12), switched from sulfonylurea (SU, n=13), switched from DPP4i (n=11), and switched from thiazolidinedione (TZD, n=37). All the participants had already used MET for their regimen.
RESULTS
Treatment with DAPA reduced HbA1c level by 1.2%±0.8%. Moreover, a significant decrease was observed in all subgroups: add-on to MET, −1.2%±0.7%; add-on to MET+DPP4i, −1.4%±0.8%; switched from SU, −1.4%±0.7%; switched from DPP4i, −0.5%±0.7%; and switched from TZD, −1.2%±0.9% (P<0.01). A significant decrease in body weight (−3.1±2.6 kg, P<0.001) was observed after DAPA administration. Estimated glomerular filtration rate and urine microalbumin were significantly decreased after 6 months of treatment with DAPA (−4.0±13.5 mL/min/1.73 m2, P=0.03; −23.6±45.9 mg/L, P<0.001).
CONCLUSION
Treatment with DAPA, whether added to or switched from other glucose-lowering agents, significantly decreased HbA1c levels in Korean patients with type 2 diabetes who visited a single primary diabetes clinic. DAPA can be considered as an optimal second-line treatment for patients with type 2 diabetes, as supported by real-world evidence studies.
Keyword
MeSH Terms
Figure
Reference
-
1. International Diabetes Federation. Key messages [Internet]. Brussels: IDF;2019. cited 2019 Feb 5. Available from: http://diabetesatlas.org/key-messages.html.2. Korean Diabetes Association. Diabetes fact sheet in Korea 2018 (ver. English) [Internet]. Seoul: KDA;2018. cited 2019 Feb 5. Available from: http://www.diabetes.or.kr/pro/news/admin.php?category=A&code=admin&number=1615&mode=view.3. American Diabetes Association. Standards of medical care in diabetes: 2012. Diabetes Care. 2012; 35(Suppl 1):S11–S63. PMID: 22187469.4. Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018; 41:2669–2701. PMID: 30291106.
Article5. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016; 134:752–772. PMID: 27470878.6. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017; 377:644–657. PMID: 28605608.
Article7. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–2128. PMID: 26378978.
Article8. Kosiborod M, Lam CSP, Kohsaka S, Kim DJ, Karasik A, Shaw J, et al. Cardiovascular events associated with sglt-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol. 2018; 71:2628–2639. PMID: 29540325.9. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380:347–357. PMID: 30415602.
Article10. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019; 393:31–39. PMID: 30424892.
Article11. Perrone-Filardi P, Avogaro A, Bonora E, Colivicchi F, Fioretto P, Maggioni AP, et al. Mechanisms linking empagliflozin to cardiovascular and renal protection. Int J Cardiol. 2017; 241:450–456. PMID: 28395981.
Article12. U.S. Food and Drug Administration. FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes [Internet]. Silver Spring: FDA;2018. cited 2019 Feb 5. Available from: https://www.fda.gov/Drugs/DrugSafety/ucm617360.htm.13. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604–612. PMID: 19414839.
Article14. Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013; 11:43. PMID: 23425012.
Article15. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010; 375:2223–2233. PMID: 20609968.
Article16. Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract. 2012; 66:446–456. PMID: 22413962.
Article17. Jabbour SA, Hardy E, Sugg J, Parikh S. Study 10 Group. Dapagliflozin is effective as add-on therapy to sitagliptin with or without metformin: a 24-week, multicenter, randomized, double-blind, placebo-controlled study. Diabetes Care. 2014; 37:740–750. PMID: 24144654.
Article18. Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012; 35:1473–1478. PMID: 22446170.
Article19. Yagi S, Aihara KI, Kondo T, Kurahashi K, Yoshida S, Endo I, et al. Predictors for the treatment effect of sodium glucose co-transporter 2 inhibitors in patients with type 2 diabetes mellitus. Adv Ther. 2018; 35:124–134. PMID: 29185199.
Article20. Bolinder J, Ljunggren O, Johansson L, Wilding J, Langkilde AM, Sjostrom CD, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014; 16:159–169. PMID: 23906445.
Article21. Wilding J, Bailey C, Rigney U, Blak B, Beekman W, Emmas C. Glycated hemoglobin, body weight and blood pressure in type 2 diabetes patients initiating dapagliflozin treatment in primary care: a retrospective study. Diabetes Ther. 2016; 7:695–711. PMID: 27585582.
Article22. Iemitsu K, Iizuka T, Takihata M, Takai M, Nakajima S, Minami N, et al. Factors influencing changes in hemoglobin a1c and body weight during treatment of type 2 diabetes with ipragliflozin: interim analysis of the ASSIGN-K study. J Clin Med Res. 2016; 8:373–378. PMID: 27081422.
Article23. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010; 33:2217–2224. PMID: 20566676.24. Matthaei S, Bowering K, Rohwedder K, Grohl A, Parikh S. Study 05 Group. Dapagliflozin improves glycemic control and reduces body weight as add-on therapy to metformin plus sulfonylurea: a 24-week randomized, double-blind clinical trial. Diabetes Care. 2015; 38:365–372. PMID: 25592197.
Article25. Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011; 13:928–938. PMID: 21672123.
Article26. Sugiyama S, Jinnouchi H, Kurinami N, Hieshima K, Yoshida A, Jinnouchi K, et al. Dapagliflozin reduces fat mass without affecting muscle mass in type 2 diabetes. J Atheroscler Thromb. 2018; 25:467–476. PMID: 29225209.
Article27. Bouchi R, Terashima M, Sasahara Y, Asakawa M, Fukuda T, Takeuchi T, et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: a pilot study. Cardiovasc Diabetol. 2017; 16:32. PMID: 28253918.
Article28. Scheerer MF, Rist R, Proske O, Meng A, Kostev K. Changes in HbA1c, body weight, and systolic blood pressure in type 2 diabetes patients initiating dapagliflozin therapy: a primary care database study. Diabetes Metab Syndr Obes. 2016; 9:337–345. PMID: 27822077.
Article29. Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ. 1998; 316:823–828. PMID: 9549452.
Article30. Cherney DZI, Cooper ME, Tikkanen I, Pfarr E, Johansen OE, Woerle HJ, et al. Pooled analysis of phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 2018; 93:231–244. PMID: 28860019.31. Ptaszynska A, Hardy E, Johnsson E, Parikh S, List J. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med. 2013; 125:181–189.
Article32. Storgaard H, Gluud LL, Bennett C, Grondahl MF, Christensen MB, Knop FK, et al. Benefits and harms of sodium-glucose co-transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2016; 11:e0166125. PMID: 27835680.
Article33. Kaku K, Kiyosue A, Inoue S, Ueda N, Tokudome T, Yang J, et al. Efficacy and safety of dapagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise. Diabetes Obes Metab. 2014; 16:1102–1110. PMID: 24909293.
Article34. Yabe D, Seino Y, Fukushima M, Seino S. β Cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015; 15:602. PMID: 25944304.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical studies of dapagliflozin in pediatric patients: a rapid review
- Fournier Gangrene in a Patient With Type 2 Diabetes Mellitus Treated With Dapagliflozin: A Case Report
- Efficacy of Body Weight Reduction on the SGLT2 Inhibitor in People with Type 2 Diabetes Mellitus
- Dapagliflozin's Effects on Glycemia and Cardiovascular Risk Factors and Incidence of Adverse Events in Patients with Type 2 Diabetes
- Comparisons of Efficacy between Dapagliflozin and Sitagliptin in Combination with Metformin in Type 2 Diabetes Mellitus Patients