Clin Exp Otorhinolaryngol.
2018 Dec;11(4):224-232. 10.21053/ceo.2018.00878.
The Presence of Neural Stem Cells and Changes in Stem Cell-Like Activity With Age in Mouse Spiral Ganglion Cells In Vivo and In Vitro
- Affiliations
-
- 1Department of Stem Cell Biology and Regenerative Medicine, Broad Center for Regenerative Medicine and Stem Cell Research, Keck School of Medicine of the University of Southern California, Los Angeles, CA, USA. wangelu@usc.edu
- 2Department of Neurosurgery, Keck School of Medicine of the University of Southern California, Los Angeles, CA, USA.
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. dzness@amc.seoul.kr
- KMID: 2439848
- DOI: http://doi.org/10.21053/ceo.2018.00878
Abstract
OBJECTIVES
Spiral ganglion neurons (SGNs) include potential endogenous progenitor populations for the regeneration of the peripheral auditory system. However, whether these populations are present in adult mice is largely unknown. We examined the presence and characteristics of SGN-neural stem cells (NSCs) in mice as a function of age.
METHODS
The expression of Nestin and Ki67 was examined in sequentially dissected cochlear modiolar tissues from mice of different ages (from postnatal day to 24 weeks) and the sphere-forming populations from the SGNs were isolated and differentiated into different cell types.
RESULTS
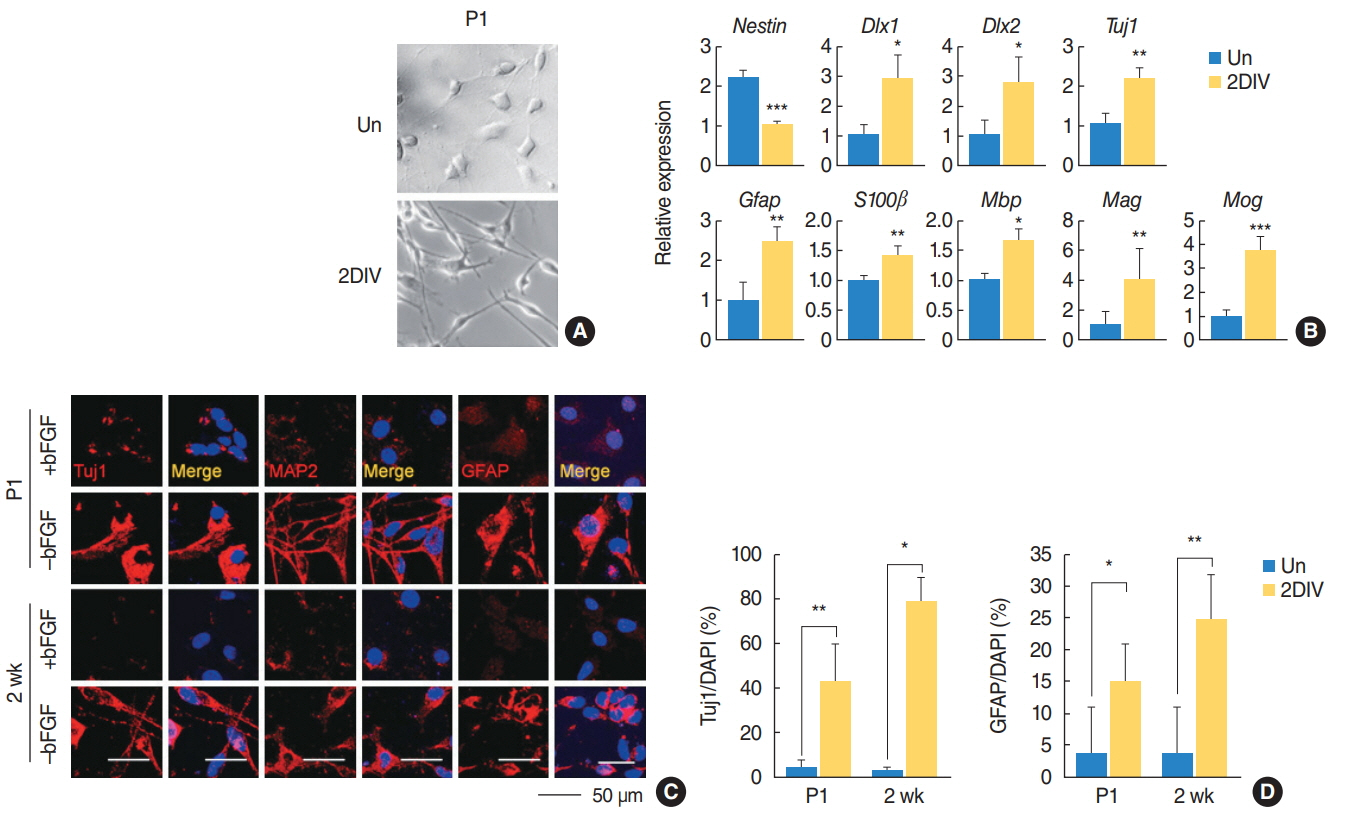
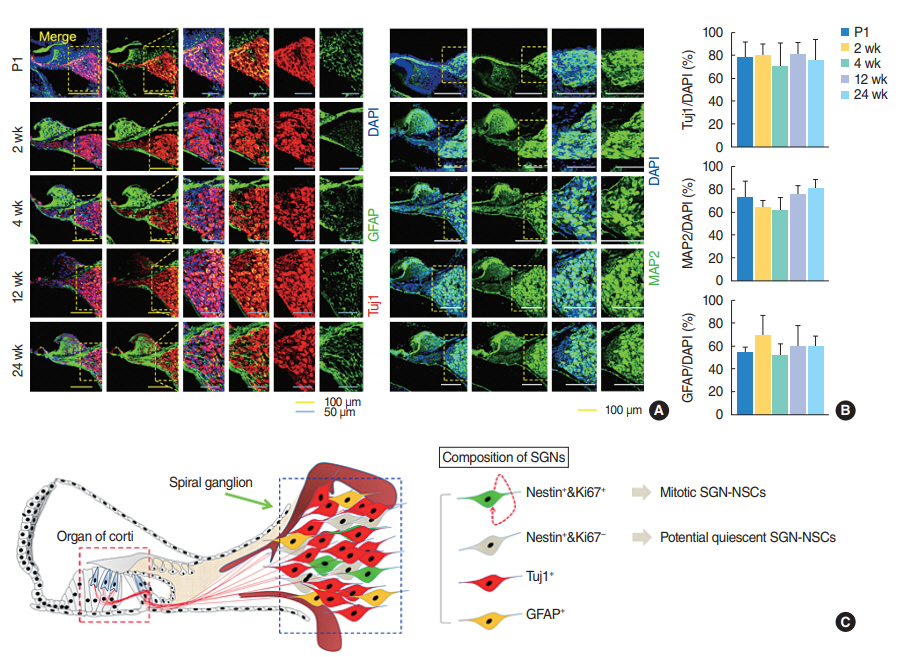
There were significant decreases in Nestin and Ki67 double-positive mitotic progenitor cells in vivo with increasing mouse age. The SGNs formed spheres exhibiting self-renewing activity and multipotent capacity, which were seen in NSCs and were capable of differentiating into neuron and glial cell types. The SGN spheres derived from mice at an early age (postnatal day or 2 weeks) contained more mitotic stem cells than those from mice at a late age.
CONCLUSION
Our findings showed the presence of self-renewing and proliferative subtypes of SGN-NSCs which might serve as a promising source for the regeneration of auditory neurons even in adult mice.
Keyword
MeSH Terms
Figure
Reference
-
1. Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009; Nov. 29(45):14077–85.
Article2. Hoeffding V, Feldman ML. Changes with age in the morphology of the cochlear nerve in rats: light microscopy. J Comp Neurol. 1988; Oct. 276(4):537–46.
Article3. Roehm PC, Hansen MR. Strategies to preserve or regenerate spiral ganglion neurons. Curr Opin Otolaryngol Head Neck Surg. 2005; Oct. 13(5):294–300.
Article4. Shi F, Edge AS. Prospects for replacement of auditory neurons by stem cells. Hear Res. 2013; Mar. 297:106–12.
Article5. Reyes JH, O’Shea KS, Wys NL, Velkey JM, Prieskorn DM, Wesolowski K, et al. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. J Neurosci. 2008; Nov. 28(48):12622–31.
Article6. Zhang L, Jiang H, Hu Z. Concentration-dependent effect of nerve growth factor on cell fate determination of neural progenitors. Stem Cells Dev. 2011; Oct. 20(10):1723–31.
Article7. Li X, Aleardi A, Wang J, Zhou Y, Andrade R, Hu Z. Differentiation of spiral ganglion-derived neural stem cells into functional synaptogenetic neurons. Stem Cells Dev. 2016; May. 25(10):803–13.
Article8. Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Geleoc GS, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007; Mar. 8(1):18–31.
Article9. Rask-Andersen H, Bostrom M, Gerdin B, Kinnefors A, Nyberg G, Engstrand T, et al. Regeneration of human auditory nerve: in vitro/in video demonstration of neural progenitor cells in adult human and guinea pig spiral ganglion. Hear Res. 2005; May. 203(1-2):180–91.
Article10. Diensthuber M, Zecha V, Wagenblast J, Arnhold S, Stover T. Clonal colony formation from spiral ganglion stem cells. Neuroreport. 2014; Oct. 25(14):1129–35.
Article11. Moon BS, Bai J, Cai M, Liu C, Shi J, Lu W. Kruppel-like factor 4-dependent Staufen1-mediated mRNA decay regulates cortical neurogenesis. Nat Commun. 2018; Jan. 9(1):401.
Article12. Moon BS, Kim HY, Kim MY, Yang DH, Lee JM, Cho KW, et al. Sur8/Shoc2 involves both inhibition of differentiation and maintenance of self-renewal of neural progenitor cells via modulation of extracellular signal-regulated kinase signaling. Stem Cells. 2011; Feb. 29(2):320–31.
Article13. Moon BS, Yun HM, Chang WH, Steele BH, Cai M, Choi SH, et al. Smek promotes corticogenesis through regulating Mbd3’s stability and Mbd3/NuRD complex recruitment to genes associated with neurogenesis. PLoS Biol. 2017; May. 15(5):e2001220.
Article14. Kamiya K, Takahashi K, Kitamura K, Momoi T, Yoshikawa Y. Mitosis and apoptosis in postnatal auditory system of the C3H/He strain. Brain Res. 2001; May. 901(1-2):296–302.
Article15. Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967; Suppl 220:1–44.16. Tinnemans MM, Lenders MH, ten Velde GP, Wagenaar SS, Blijham GH, Ramaekers FC, et al. Evaluation of proliferation parameters in in vivo bromodeoxyuridine labelled lung cancers. Virchows Arch. 1995; 427(3):295–301.
Article17. Kempermann G, Gage FH, Aigner L, Song H, Curtis MA, Thuret S, et al. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018; Jul. 23(1):25–30.
Article18. Tamura T, Nakagawa T, Iguchi F, Tateya I, Endo T, Kim TS, et al. Transplantation of neural stem cells into the modiolus of mouse cochleae injured by cisplatin. Acta Otolaryngol Suppl. 2004; Mar. (551):65–8.
Article19. Hu Z, Andang M, Ni D, Ulfendahl M. Neural cograft stimulates the survival and differentiation of embryonic stem cells in the adult mammalian auditory system. Brain Res. 2005; Jul. 1051(1-2):137–44.
Article20. Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013; Jun. 78(5):785–98.
Article21. Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010; Feb. 463(7284):1035–41.
Article22. Khan AM, Handzel O, Burgess BJ, Damian D, Eddington DK, Nadol JB Jr. Is word recognition correlated with the number of surviving spiral ganglion cells and electrode insertion depth in human subjects with cochlear implants. Laryngoscope. 2005; Apr. 115(4):672–7.
Article23. Fayad JN, Linthicum FH Jr. Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006; Aug. 116(8):1310–20.
Article24. Nadol JB Jr, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989; Jun. 98(6):411–6.
Article25. Lousteau RJ. Increased spiral ganglion cell survival in electrically stimulated, deafened guinea pig cochleae. Laryngoscope. 1987; Jul. 97(7 Pt 1):836–42.
Article26. Kanzaki S, Stover T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, et al. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002; Dec. 454(3):350–60.
Article