J Breast Cancer.
2017 Sep;20(3):270-278. 10.4048/jbc.2017.20.3.270.
Development of a Nomogram to Predict N2 or N3 Stage in T1–2 Invasive Breast Cancer Patients with No Palpable Lymphadenopathy
- Affiliations
-
- 1Division of Breast and Endocrine Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. seokwon1.kim@samsung.com
- KMID: 2438994
- DOI: http://doi.org/10.4048/jbc.2017.20.3.270
Abstract
- PURPOSE
Subsequent to the American College of Surgeons Oncology Group (ACOSOG) Z0011 and After Mapping of the Axilla: Radiotherapy or Surgery (AMAROS) trials, complete axillary lymph node dissection is not routinely performed, even in cases where metastatic sentinel lymph nodes are detected. We investigated the percentage of N2 or N3 stages in T1-2 invasive breast cancer patients with no lymphadenopathy and developed a nomogram to predict the possibility of N2 or N3 stages in these patients.
METHODS
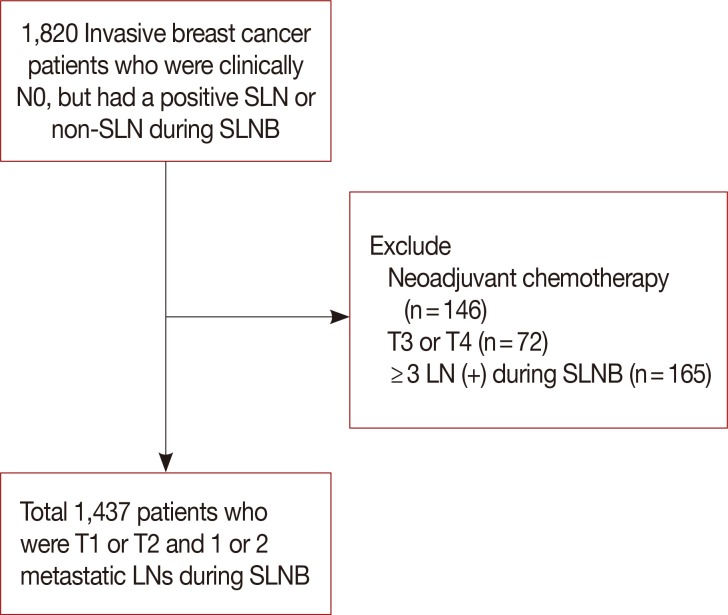
We retrospectively reviewed the charts of invasive breast cancer patients who were clinically N0 stage, but had a positive sentinel or non-sentinel lymph node detected on sentinel lymph node biopsy. The association of potential risk factors with known outcomes (N2 or N3 stages) was tested using logistic regression analysis. Variables with p<0.05 in the multivariate analysis were included in the nomogram. Internal performance validation was carried out using a 5-fold cross validation method.
RESULTS
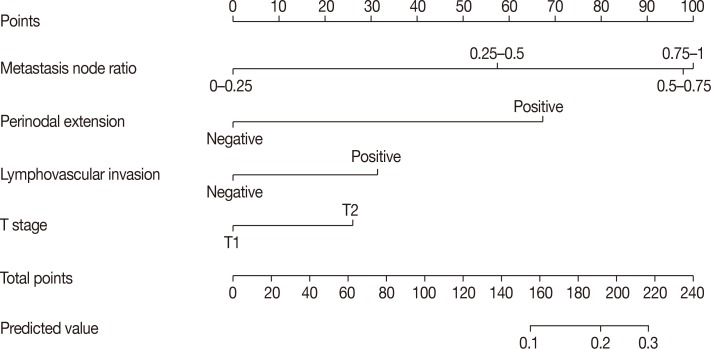
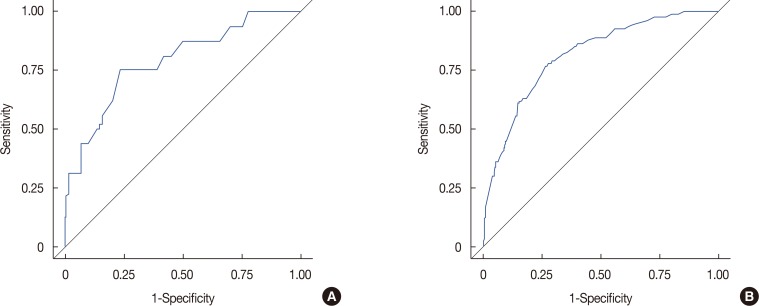
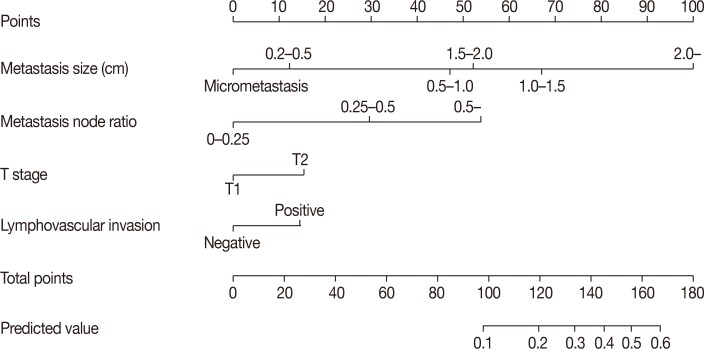
Among a total of 1,437 patients, 1,355 patients had stage N1 disease (94.3%), while 82 had stage N2 or N3 disease (5.7%). Multivariate stepwise logistic regression analysis revealed lymphovascular invasion (p=0.008), T2 stage (p=0.026), metastatic lymph node ratio (p<0.001), and perinodal extension (p<0.001) as independent predictors of N2 or N3 stages. A nomogram was developed based on these factors. The area under the curve estimated from the receiver operating characteristic graph was 0.8050 in the model set and 0.8246 in the test set.
CONCLUSION
Our nomogram can be employed for the prediction of N2 or N3 stage among cases fulfilling the ACOSOG Z0011 or AMAROS criteria.
MeSH Terms
Figure
Cited by 1 articles
-
Patterns of Axillary Lymph Node Metastasis in Breast Cancer: A Prospective Single-Center Study
Hee Jun Choi, Jae-Myung Kim, Jai Min Ryu, Isaac Kim, Seok Jin Nam, Jonghan Yu, Se Kyung Lee, Jeong Eon Lee, Seok Won Kim
J Breast Cancer. 2018;21(4):447-452. doi: 10.4048/jbc.2018.21.e50.
Reference
-
1. Bevilacqua JL, Kattan MW, Fey JV, Cody HS 3rd, Borgen PI, Van Zee KJ. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol. 2007; 25:3670–3679. PMID: 17664461.
Article2. Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010; 252:426–432. PMID: 20739842.3. Donker M, van Tienhoven G, Straver ME, Meijnen P, van de, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014; 15:1303–1310. PMID: 25439688.
Article4. Esteva FJ, Hortobagyi GN. Locally advanced breast cancer. Hematol Oncol Clin North Am. 1999; 13:457–472. PMID: 10363140.
Article5. Han A, Moon HG, Kim J, Ahn SK, Park IA, Han W, et al. Reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2013; 16:378–385. PMID: 24454459.
Article6. Lee Y, Lee CK. Classification of multiple cancer types by multicategory support vector machines using gene expression data. Bioinformatics. 2003; 19:1132–1139. PMID: 12801874.
Article7. Katz A, Smith BL, Golshan M, Niemierko A, Kobayashi W, Raad RA, et al. Nomogram for the prediction of having four or more involved nodes for sentinel lymph node-positive breast cancer. J Clin Oncol. 2008; 26:2093–2098. PMID: 18445838.
Article8. Unal B, Gur AS, Beriwal S, Tang G, Johnson R, Ahrendt G, et al. Predicting likelihood of having four or more positive nodes in patient with sentinel lymph node-positive breast cancer: a nomogram validation study. Int J Radiat Oncol Biol Phys. 2009; 75:1035–1040. PMID: 19327916.
Article9. Harlow SP, Krag DN, Julian TB, Ashikaga T, Weaver DL, Feldman SA, et al. Prerandomization Surgical Training for the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial: a randomized phase III clinical trial to compare sentinel node resection to conventional axillary dissection in clinically node-negative breast cancer. Ann Surg. 2005; 241:48–54. PMID: 15621990.10. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007; 8:881–888. PMID: 17851130.
Article11. Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg. 2016; 264:413–420. PMID: 27513155.12. Caudle AS, Hunt KK, Kuerer HM, Meric-Bernstam F, Lucci A, Bedrosian I, et al. Multidisciplinary considerations in the implementation of the findings from the American College of Surgeons Oncology Group (ACOSOG) Z0011 study: a practice-changing trial. Ann Surg Oncol. 2011; 18:2407–2412. PMID: 21327455.
Article13. Leitch AM, McCall L, Beitsch P, Whitworth P, Reintgen D, Blumencranz P, et al. Factors influencing accrual to ACOSOG Z0011, a randomized phase III trial of axillary dissection vs. observation for sentinel node positive breast cancer. J Clin Oncol. 2006; 24:601.
Article14. Gatzemeier W, Mann GB. Which sentinel lymph-node (SLN) positive breast cancer patient needs an axillary lymph-node dissection (ALND): ACOSOG Z0011 results and beyond. Breast. 2013; 22:211–216. PMID: 23478200.15. Van Zee KJ, Manasseh DM, Bevilacqua JL, Boolbol SK, Fey JV, Tan LK, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003; 10:1140–1151. PMID: 14654469.
Article16. Scomersi S, Da Pozzo F, Torelli L, Zanconati F, Tonutti M, Dore F, et al. Clinicopathologic factors predicting involvement of nonsentinel axillary lymphnodes in breast cancer patients: is axillary dissection always indicated? Ann Ital Chir. 2010; 81:335–341. PMID: 21294386.17. Hwang RF, Krishnamurthy S, Hunt KK, Mirza N, Ames FC, Feig B, et al. Clinicopathologic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Oncol. 2003; 10:248–254. PMID: 12679309.
Article18. Schrenk P. Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Eur Surg. 2005; 37:175–177.
Article19. Straver ME, Meijnen P, van Tienhoven G, van de, Mansel RE, Bogaerts J, et al. Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial. Ann Surg Oncol. 2010; 17:1854–1861. PMID: 20300966.
Article20. Chang RF, Wu WJ, Moon WK, Chou YH, Chen DR. Support vector machines for diagnosis of breast tumors on US images. Acad Radiol. 2003; 10:189–197. PMID: 12583571.
Article21. Dreiseitl S, Ohno-Machado L, Kittler H, Vinterbo S, Billhardt H, Binder M. A comparison of machine learning methods for the diagnosis of pigmented skin lesions. J Biomed Inform. 2001; 34:28–36. PMID: 11376540.
Article22. Dreiseitl S, Ohno-Machado L. Logistic regression and artificial neural network classification models: a methodology review. J Biomed Inform. 2002; 35:352–359. PMID: 12968784.
Article23. Viale G, Maiorano E, Pruneri G, Mastropasqua MG, Valentini S, Galimberti V, et al. Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Ann Surg. 2005; 241:319–325. PMID: 15650643.
Article24. Usami S, Moriya T, Kasajima A, Suzuki A, Ishida T, Sasano H, et al. Pathological aspects of core needle biopsy for non-palpable breast lesions. Breast Cancer. 2005; 12:272–278. PMID: 16286907.
Article25. Harris GC, Denley HE, Pinder SE, Lee AH, Ellis IO, Elston CW, et al. Correlation of histologic prognostic factors in core biopsies and therapeutic excisions of invasive breast carcinoma. Am J Surg Pathol. 2003; 27:11–15. PMID: 12502923.
Article26. van der Loo EM, Sastrowijoto SH, Bril H, van Krimpen C, de Graaf PW, Eulderink F. Less operations required due to perioperative frozen section examination of sentinel nodes in 275 breast cancer patients. Ned Tijdschr Geneeskd. 2001; 145:1986–1991. PMID: 11680071.27. Cheng L, Pisansky TM, Ramnani DM, Leibovich BC, Cheville JC, Slezak J, et al. Extranodal extension in lymph node-positive prostate cancer. Mod Pathol. 2000; 13:113–118. PMID: 10697266.
Article28. Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007; 25:3657–3663. PMID: 17485711.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Development of a Nomogram to Predict N2 or N3 Stage in T1-2 Invasive Breast Cancer Patients with No Palpable Lymphadenopathy
- Preoperative Staging in Non-Small Cell Lung Cancer without Lymphadenopathy on Computed Tomogram
- A Novel Prognostic Nomogram for Predicting Risks of Distant Failure in Patients with Invasive Breast Cancer Following Postoperative Adjuvant Radiotherapy
- Development and Validation of Web-Based Nomograms to Predict Postoperative Invasive Component in Ductal Carcinoma in Situ at Needle Breast Biopsy
- A Clinical Analysis of the Characteristics of the Lymph Node Metastasis in Patients with Gastrics Cancer