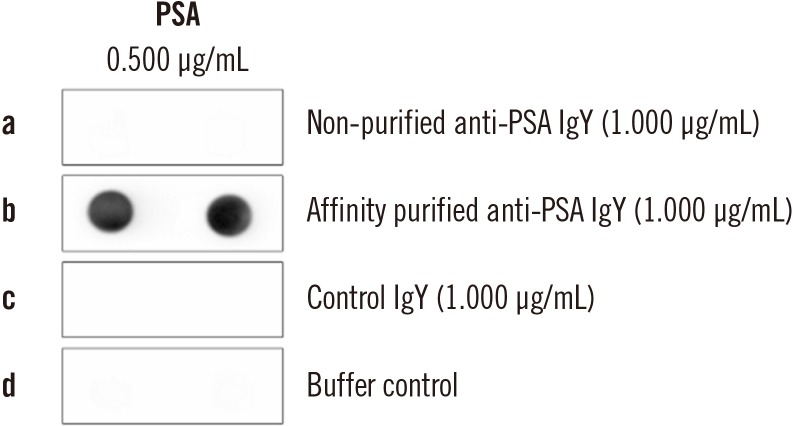
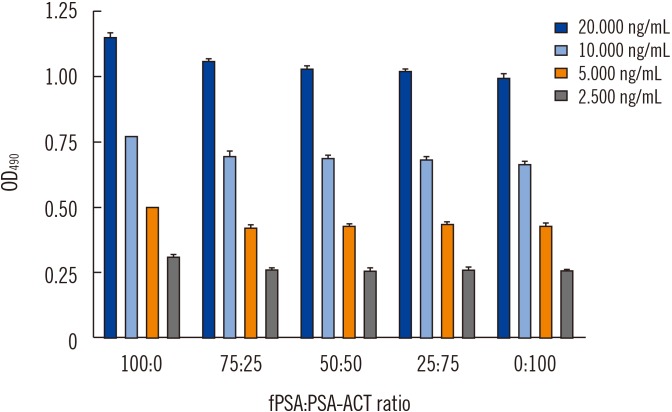
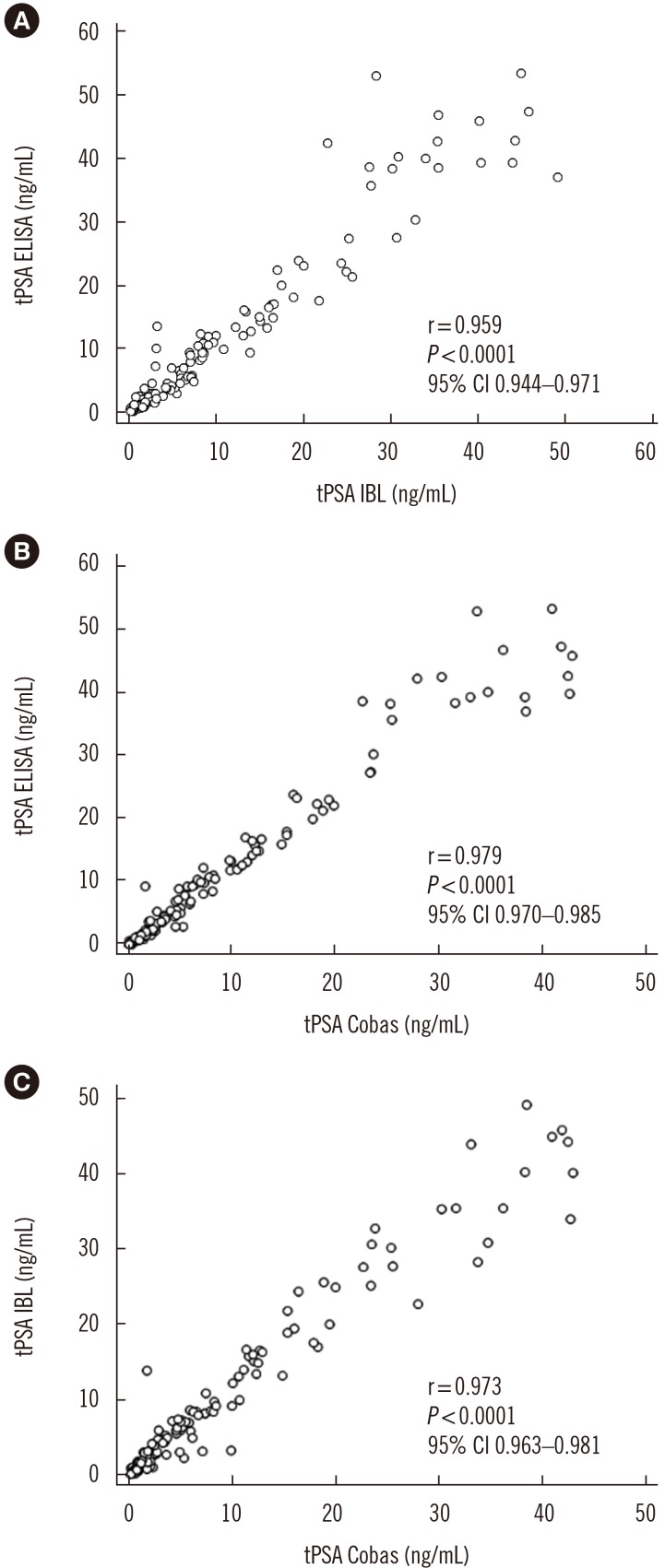
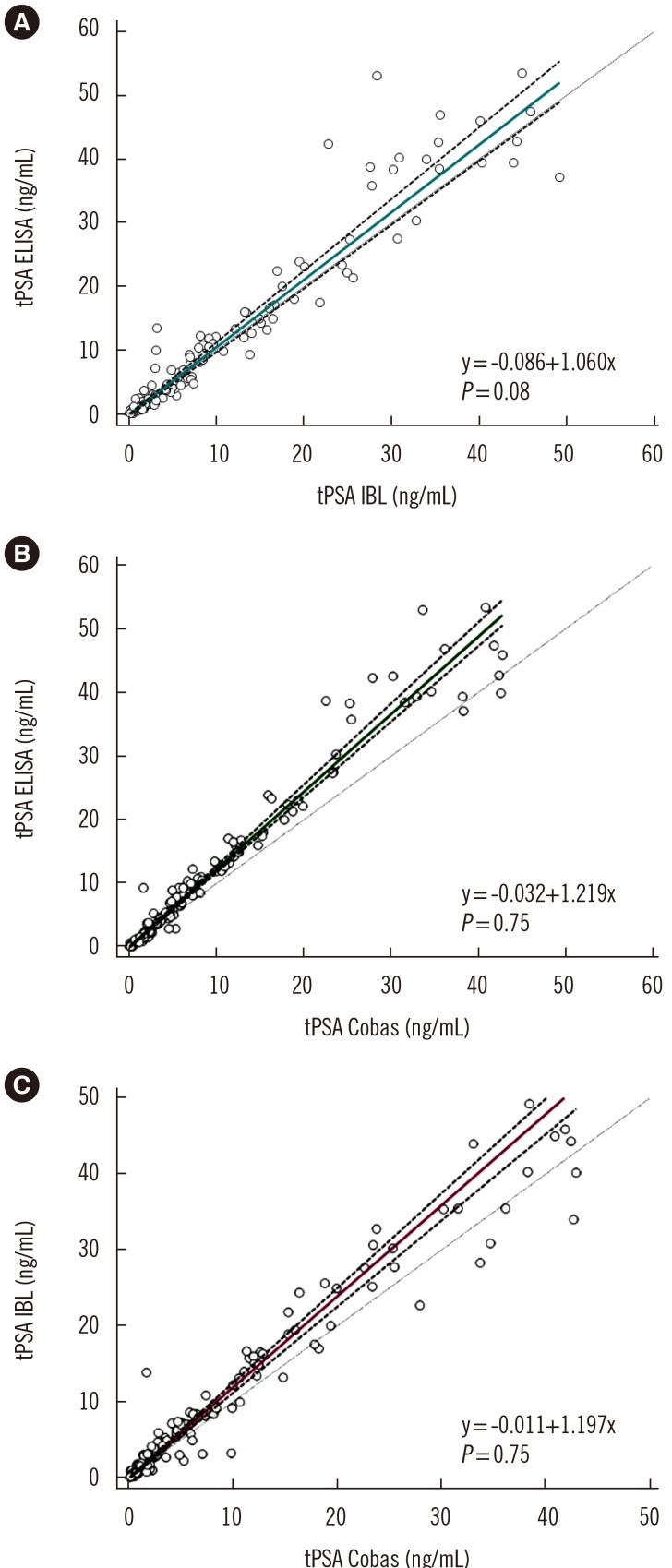
Ann Lab Med.
2019 Jul;39(4):373-380. 10.3343/alm.2019.39.4.373.
Development and Evaluation of an Immunoglobulin Y-Based ELISA for Measuring Prostate Specific Antigen in Human Serum
- Affiliations
-
- 1Faculty of Chemistry, Division of Medicinal Chemistry and Microbiology, Wrocław University of Science and Technology, Wrocław, Poland. marcin.sienczyk@pwr.edu.pl
- 2Greater Poland Cancer Centre, Department of Laboratory Diagnostics, Poznań, Poland.
- KMID: 2438884
- DOI: http://doi.org/10.3343/alm.2019.39.4.373
Abstract
- BACKGROUND
Measurement of serum prostate specific antigen (PSA) concentrations remains one of the leading methods for diagnosing prostate cancer. We developed and evaluated an immunoglobulin Y (IgY)-based ELISA to measure total PSA (tPSA) concentrations in human serum that could be used as an alternative to commercially available in vitro diagnostic assays that rely on mouse monoclonal IgG.
METHODS
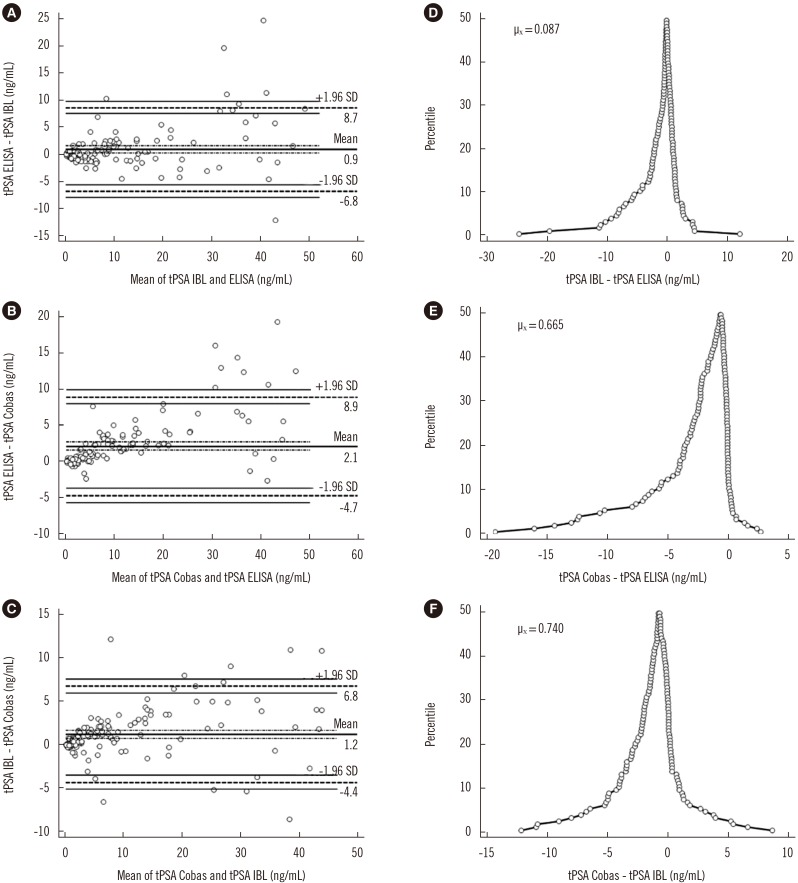
A sandwich ELISA based on an anti-PSA IgY antibody was developed. We evaluated the ability of the anti-PSA IgY antibody to detect free and complexed PSA at the same molar ratio. The assay was optimized, and its analytical performance was verified by calculating limit of background (LoB), limit of detection (LoD), and limit of quantification (LoQ). We performed correlation and regression analyses between tPSA concentrations measured by our ELISA and those from commercial assays: Cobas 6000 (Roche Diagnostics, Warszawa, Poland) and PSA total ELISA (IBL International, Hamburg, Germany).
RESULTS
LoB, LoD, and LoQ, were 0.061, 0.083, and 0.100 ng/mL, respectively, and linearity range was 0.100-3.375 ng/mL. tPSA concentrations from our IgY-based ELISA strongly correlated with those from the commercial assays.
CONCLUSIONS
Our IgY-based ELISA is an efficient equivalent to the above commercial assays. The use of IgY as the detecting agent could reduce the risk of false positive results, as well as decrease the overall cost of analysis.
Keyword
MeSH Terms
Figure
Reference
-
1. Davis SN, Diefenbach MA, Valdimarsdottir H, Chen T, Hall SJ, Thompson HS. Pros and cons of prostate cancer screening: associations with screening knowledge and attitudes among urban African American men. J Natl Med Assoc. 2010; 102:174–182. PMID: 20355346.2. Parker C, Gillessen S, Heidenreich A, Horwich A. ESMO Guidelines Committee. Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015; 26(S5):v69–v77. PMID: 26205393.3. Sosnowski R, Zagrodzka M, Borkowski T. The limitations of multiparametric magnetic resonance imaging also must be borne in mind. Cent European J Urol. 2016; 69:22–23.4. Shariat S, Karakiewicz PI. Screening for prostate cancer in 2007: The PSA era and its challenges are not over. Eur Urol. 2008; 53:457–460. PMID: 18079050.5. Catalona WJ, Loeb S. Prostate cancer screening and determining the appropriate prostate-specific antigen cut-off values. J Natl Compr Canc Netw. 2010; 8:265–270. PMID: 20141681.6. Polascik TJ, Oesterling JE, Partin AW. Prostate specific antigen: a decade of discovery--what we have learned and where we are going. J Urol. 1999; 162:293–306. PMID: 10411025.7. Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol. 2003; 21:383–391. PMID: 12525533.8. Kumar A, Mikolajczyk SD, Goel AS, Millar LS, Saedi MS. Expression of pro form of prostate-specific antigen by mammalian cells and its conversion to mature, active form by human kallikrein 2. Cancer Res. 1997; 57:3111–3114. PMID: 9242434.9. Travis J, Garner D, Bowen J. Human alpha-1-antichymotrypsin: purification and properties. Biochemistry. 1978; 17:5647–5651. PMID: 103576.10. Lilja H, Christensson A, Dahlén U, Matikainen MT, Nilsson O, Pettersson K, et al. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin Chem. 1991; 37:1618–1625. PMID: 1716536.11. Jung K, Elgeti U, Lein M, Brux B, Sinha P, Rudolph B, et al. Ratio of free or complexed prostate-specific antigen (PSA) to total PSA: which ratio improves differentiation between benign prostatic hyperplasia and prostate cancer? Clin Chem. 2000; 46:55–62. PMID: 10620572.12. Catalona WJ, Partin AW, Slawin KM, Brawer MK, Flanigan RC, Patel A, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998; 279:1542–1547. PMID: 9605898.13. Prcic A, Begic E, Hiros M. Actual contribution of free to total PSA ratio in prostate diseases differentiation. Med Arch. 2016; 70:288–292. PMID: 27703291.14. Filella X, Giménez N. Evaluation of [−2] proPSA and Prostate Health Index (phi) for the detection of prostate cancer: a systematic review and meta-analysis. Clin Chem Lab Med. 2013; 51:729–739. PMID: 23154423.15. Loeb S, Catalona WJ. The Prostate Health Index: a new test for the detection of prostate cancer. Ther Adv Urol. 2014; 6:74–77. PMID: 24688603.16. Łupicka-Słowik A, Walczak M, Grzywa R, Bobrek K, Łęcka M, Boivin S, et al. Generation and application of polyclonal IgY antibodies specific for full-length and nicked prostate-specific antigen. Bioanalysis. 2014; 6:3197–3213. PMID: 25529887.17. Schiettecatte J, Anckaert E, Smitz J. Interferences in immunoassays. In : Chiu NHL, editor. Advances in immunoassay technology. Rijeka: InTech;2012. p. 45–62.18. Larsson A, Mellstedt H. Chicken antibodies: a tool to avoid interference by human anti-mouse antibodies in ELISA after in vivo treatment with murine monoclonal antibodies. Hybridoma. 1992; 11:33–39. PMID: 1737638.19. Larsson A, Wejåker PE, Forsberg PO, Lindahl T. Chicken antibodies: a tool to avoid interference by complement activation in ELISA. J Immunol Methods. 1992; 156:79–83. PMID: 1431165.20. Wang ZQ, Fu GY, Jing FJ, Jin J, Ren HJ, Jiang P, et al. Detection of Trichinella spiralis circulating antigens in serum of experimentally infected mice by an IgY-mAb sandwich ELISA. Foodborne Pathog Dis. 2012; 9:727–733. PMID: 22730960.21. Leenaars PP, Hendriksen CF, de Leeuw WA, Carat F, Delahaut P, Fischer R, et al. The production of polyclonal antibodies in laboratory animals. The report and recommendations of ECVAM Workshop 35. Altern Lab Anim. 1999; 27:79–102. PMID: 25423403.22. Polson A, von Wechmar MB, van Regenmortel MH. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol Commun. 1980; 9:475–493. PMID: 7429529.23. Łupicka-Słowik A, Psurski M, Grzywa R, Bobrek K, Smok P, Walczak M. Development of adenosine deaminase-specific IgY antibodies: Diagnostic and inhibitory application. Appl Biochem Biotechnol. 2018; 184:1358–1374. PMID: 29043661.24. Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008; 29:S49–S52. PMID: 18852857.25. Armitage P, Berry G, Matthews JNS. Statistical methods in medical research. Fourth Edition. Blackwell Science;2002. p. 187–207.26. Lin LIK. A Concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989; 45:255–268. PMID: 2720055.27. Passing H. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in Clinical Chemistry, Part 1. Clin Chem Clin Biochem. 1983; 21:709–720.28. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999; 8:135–160. PMID: 10501650.29. Krouwer JS, Monti KL. A simple, graphical method to evaluate laboratory assays. Eur J Clin Chem Clin Biochem. 1995; 33:525–527. PMID: 8547437.30. Semjonow A, de Angelis G, Oberpenning F, Schmidt HP, Brandt B, Hertle L. The clinical impact of different assays for prostate specific antigen. BJU Int. 2000; 86:590–597. PMID: 10971300.31. Carsten S, Klaas M, Müller C, Schnorr D, Loening SA, Jung K. Interchangeability of measurements of total and free prostate-specific antigen in serum with 5 frequently used assay combinations: an update. Clin Chem. 2006; 52:59–64. PMID: 16391327.32. Kort SAR, Martens F, Vanpoucke H, van Duijnhoven HL, Blankenstein MA. Comparison of 6 automated assays for total and free prostate-specific antigen with special reference to their reactivity toward the WHO 96/670 reference preparation. Clin Chem. 2006; 52:1568–1574. PMID: 16762996.33. Murthy V, Rishi A, Gupta S, Kannan S, Mahantshetty U, Tongaonkar H, et al. Clinical impact of prostate specific antigen (PSA) inter-assay variability on management of prostate cancer. Clin Biochem. 2016; 49:79–84. PMID: 26506115.34. Foj L, Fiella X, Alcover J, Augé JM, Escudero JM, Molina R. Variability of assay methods for total and free PSA after WHO standardization. Tumor Biol. 2014; 35:1867–1873.35. Liu LN, Jing FJ, Cui J, Fu GY, Wang ZQ. Detection of circulating antigen in serum of mice infected with Trichinella spiralis by an IgY-IgM mAb sandwich ELISA. Exp Parasitol. 2013; 133:150–155. PMID: 23183166.36. Silva DA, Silva NM, Mineo TW, Pajuaba Neto AA, Ferro EA, Mineo JR. Heterologous antibodies to evaluate the kinetics of the humoral immune response in dogs experimentally infected with Toxoplasma gondii RH strain. Vet Parasitol. 2002; 107:181–195. PMID: 12127249.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Factors Influencing the Percentage of Free Serum Prostate Specific Antigen Levels in Men without Clinically Detectable Prostate Cance
- Change of serum prostate specific antigen values after radiation therapy in prostate cancer
- The Diagnostic Value of Prostate-specific Antigen and the of Routine Laboratory Examination for Early Detection
- Study of Prostate Volume, Age, Serum Prostate Specific Antigen and Testosterone in Patients with Benign Prostate Hyperplasia with Lower Urinary Tract Symptoms
- In Search of a New Prostate-Specific Antigen