Cancer Res Treat.
2019 Jan;51(1):90-97. 10.4143/crt.2017.577.
A Randomized, Double-Blind, Placebo-Controlled Study of the Safety and Efficacy of Olanzapine for the Prevention of Chemotherapy-Induced Nausea and Vomiting in Patients Receiving Moderately Emetogenic Chemotherapy: Results of the Korean South West Oncology Group (KSWOG) Study
- Affiliations
-
- 1Department of Internal Medicine, Chonbuk National University Medical School, Jeonju, Korea. eksong@jbnu.ac.kr
- 2Research Institute of Clinical Medicine of Chonbuk National University, Biomedical Research Institute of Chonbuk National University Hospital, Jeonju, Korea.
- 3Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Korea.
- 4Department of Internal Medicine, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 5Department of Internal Medicine, Wonkwang University School of Medicine, Iksan, Korea.
- 6Department of Internal Medicine, Soonchunhyang University Hospital, Cheonan, Korea.
- 7Department of Internal Medicine, Gyeongsang National University Hospital, Gyeongsang National University School of Medicine, Jinju, Korea.
- 8Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea.
- 9Center for Clinical Pharmacology and Biomedical Research Institute, Chonbuk National University Hospital, Jeonju, Korea.
- KMID: 2437602
- DOI: http://doi.org/10.4143/crt.2017.577
Abstract
- PURPOSE
Data on the efficacy of olanzapine in patients receiving moderately emetogenic chemotherapy (MEC) are limited. This study aimed to evaluate and compare the efficacy of olanzapine versus placebo in controlling nausea and vomiting in patients receiving MEC.
MATERIALS AND METHODS
We conducted a randomized, double-blind, placebo-controlled study to determine whether olanzapine can reduce the frequency of chemotherapy-induced nausea and vomiting (CINV) and improve the quality of life (QOL) in patients receiving palonosetron and dexamethasone as prophylaxis for MEC-induced nausea and vomiting. The primary end point was complete response for the acute phase (0-24 hours after chemotherapy). The secondary end points were complete response for the delayed (24-120 hours) and overall phase (0-120 hours), proportion of significant nausea (visual analogue scale ≥ 25 mm), use ofrescue medications, and effect on QOL.
RESULTS
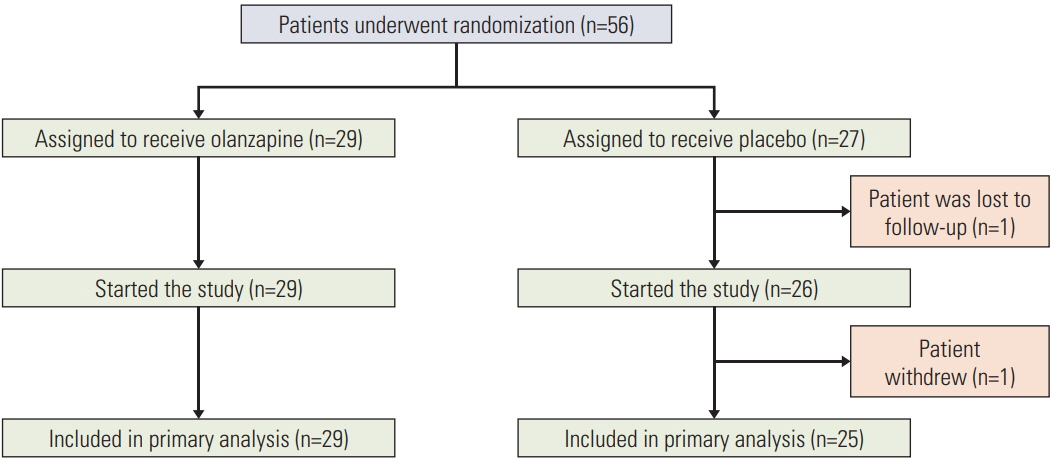
Fifty-six patients were randomized to the olanzapine (n=29) and placebo (n=27) groups. Complete response rates were not significantly different between the olanzapine and placebo groups in the acute (96.5% vs. 88.0%, p=0.326), delayed (69.0% vs. 48.0%, p=0.118), and overall phases (69.0% vs. 48.0%, p=0.118). However, the percentage of patients with significant nausea (17.2% vs. 44.0%, p=0.032) and the use of rescue medications (0.03±0.19 vs. 1.88±2.88, p=0.002) were lower in the olanzapine group than in the placebo. Furthermore, the olanzapine group demonstrated better QOL (p=0.015).
CONCLUSION
Olanzapine combined with palonosetron and dexamethasone significantly improved QOL and vomiting control among previously untreated patients receiving MEC, although the efficacy was limited to the reduction of the frequency of CINV.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Ingle RJ, Burish TG, Wallston KA. Conditionability of cancer chemotherapy patients. Oncol Nurs Forum. 1984; 11:97–102.2. Richardson JL, Marks G, Levine A. The influence of symptoms of disease and side effects of treatment on compliance with cancer therapy. J Clin Oncol. 1988; 6:1746–52.
Article3. Herrstedt J. Antiemetics: an update and the MASCC guidelines applied in clinical practice. Nat Clin Pract Oncol. 2008; 5:32–43.
Article4. Hesketh PJ, Grunberg SM, Herrstedt J, de Wit R, Gralla RJ, Carides AD, et al. Combined data from two phase III trials of the NK1 antagonist aprepitant plus a 5HT 3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: effect of gender on treatment response. Support Care Cancer. 2006; 14:354–60.5. Hesketh PJ, Bohlke K, Lyman GH, Basch E, Chesney M, Clark-Snow RA, et al. Antiemetics: American Society of Clinical Oncology focused guideline update. J Clin Oncol. 2016; 34:381–6.
Article6. Herrstedt J, Roila F, Warr D, Celio L, Navari RM, Hesketh PJ, et al. 2016 Updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following high emetic risk chemotherapy. Support Care Cancer. 2017; 25:277–88.
Article7. Jordan K, Gralla R, Jahn F, Molassiotis A. International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol. 2014; 722:197–202.
Article8. Geling O, Eichler HG. Should 5-hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol. 2005; 23:1289–94.
Article9. Bymaster FP, Falcone JF, Bauzon D, Kennedy JS, Schenck K, DeLapp NW, et al. Potent antagonism of 5-HT(3) and 5-HT(6) receptors by olanzapine. Eur J Pharmacol. 2001; 430:341–9.
Article10. Navari RM, Qin R, Ruddy KJ, Liu H, Powell SF, Bajaj M, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016; 375:134–42.
Article11. Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011; 9:188–95.
Article12. Mizukami N, Yamauchi M, Koike K, Watanabe A, Ichihara K, Masumori N, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly or moderately emetogenic chemotherapy: a randomized, double-blind, placebo-controlled study. J Pain Symptom Manage. 2014; 47:542–50.
Article13. Borjeson S, Hursti TJ, Peterson C, Fredikson M, Furst CJ, Avall-Lundqvist E, et al. Similarities and differences in assessing nausea on a verbal category scale and a visual analogue scale. Cancer Nurs. 1997; 20:260–6.14. Martin AR, Pearson JD, Cai B, Elmer M, Horgan K, Lindley C. Assessing the impact of chemotherapy-induced nausea and vomiting on patients' daily lives: a modified version of the Functional Living Index-Emesis (FLIE) with 5-day recall. Support Care Cancer. 2003; 11:522–7.15. Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017; 35:3240–61.
Article16. Berger MJ, Ettinger DS, Aston J, Barbour S, Bergsbaken J, Bierman PJ, et al. NCCN guidelines insights: antiemesis, version 2.2017. J Natl Compr Canc Netw. 2017; 15:883–93.
Article17. Chiu L, Chow R, Popovic M, Navari RM, Shumway NM, Chiu N, et al. Efficacy of olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis. Support Care Cancer. 2016; 24:2381–92.
Article18. Navari RM, Einhorn LH, Passik SD, Loehrer PJ Sr, Johnson C, Mayer ML, et al. A phase II trial of olanzapine for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier Oncology Group study. Support Care Cancer. 2005; 13:529–34.
Article19. Tan L, Liu J, Liu X, Chen J, Yan Z, Yang H, et al. Clinical research of olanzapine for prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res. 2009; 28:131.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Randomized Double-Blind, Double-Dummy, Multicenter Trial of Azasetron versus Ondansetron to Evaluate Efficacy and Safety in the Prevention of Delayed Nausea and Vomiting Induced by Chemotherapy
- Compliance with the Protocol Considered Emetogenic Potential for Prophylaxis of Chemotherapy Induced Nausea and Vomiting
- Prophylactic antiemetics therapy against gynecologic cancer chemotherapy
- Comparative study of an aprepitant regimen with an ondansetron regimen, for efficacy in gynecological cancer patients with chemotherapy
- The Meta-Analysis of the Effect of Acupressure for Nausea and Vomiting in Cancer Patients Receiving Chemotherapy