Neonatal Med.
2018 Nov;25(4):161-169. 10.5385/nm.2018.25.4.161.
Antenatal Corticosteroids and Clinical Outcomes of Preterm Singleton Neonates with Intrauterine Growth Restriction
- Affiliations
-
- 1Division of Neonatology, Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea. ljinna@snu.ac.kr
- 2Department of Biostatistics, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea.
- 3Division of Neonatology, Department of Pediatrics, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea.
- KMID: 2436134
- DOI: http://doi.org/10.5385/nm.2018.25.4.161
Abstract
- PURPOSE
We assessed the influence of antenatal corticosteroid (ACS) on the inhospital outcomes of intrauterine growth restriction (IUGR) infants.
METHODS
A retrospective study was conducted with singletons born at 23âºâ° to 33âºâ¶ weeks of gestation at Seoul National University Hospital from 2007 to 2014. We compared clinical outcomes between infants who received ACS 2 to 7 days before birth (complete ACS), at < 2 or >7 days (incomplete ACS), and those who did not receive ACS in IUGR and AGA infants. Multivariate logistic regression using Firth's penalized likelihood was performed.
RESULTS
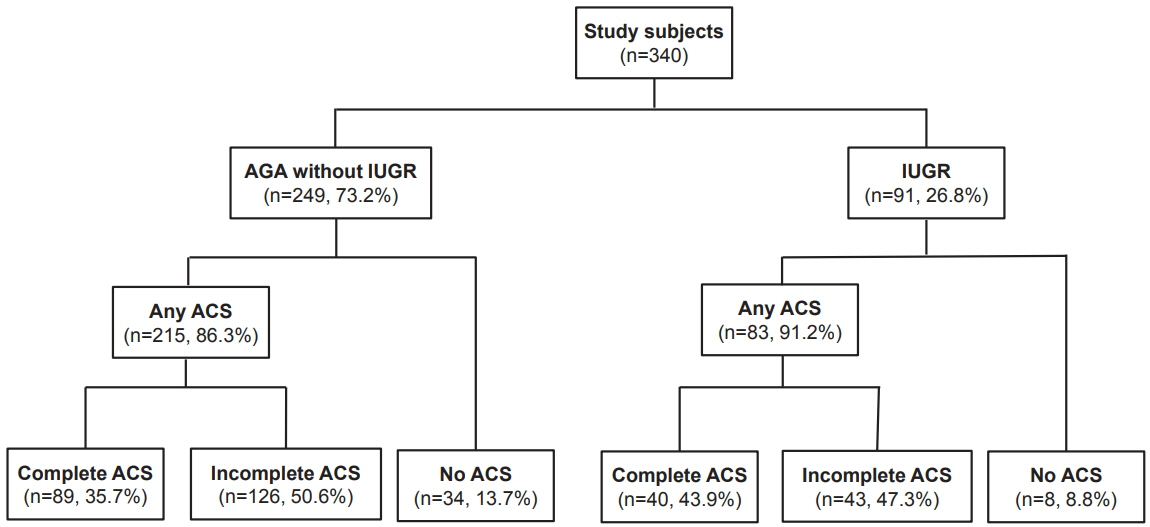
304 neonates with 91 IUGR neonates were eligible. Among AGA neonates, mortality (adjusted odds ratio [aOR], 0.13; 95% confidence interval [CI], 0.02 to 0.78), hypotension within 7 postnatal days (aOR, 0.20; 95% CI, 0.06 to 0.64), and severe bronchopulmonary dysplasia (BPD) or death (aOR, 0.24; 95% CI, 0.07 to 0.77) were lower in complete ACS group after adjusting for pregnancy induced hypertension and uncontrolled preterm labor. Mortality (aOR, 0.18; 95% CI, 0.04 to 0.78), hypotension (aOR, 0.26; 95% CI, 0.09 to 0.70), and severe BPD or death (aOR, 0.33; 95% CI, 0.12 to 0.92) were also lower in the incomplete ACS group. Among IUGR infants, after adjusting for birth weight and 5-minute Apgar score, inhaled nitric oxide use within 14 postnatal days was lower in both complete ACS (aOR, 0.07; 95% CI, 0.01 to 0.67) and incomplete ACS (aOR, 0.04; 95% CI, 0.01 to 0.37) groups.
CONCLUSION
ACS was not effective in reducing morbidities in IUGR preterm infants.
Keyword
MeSH Terms
-
Adrenal Cortex Hormones*
Apgar Score
Birth Weight
Bronchopulmonary Dysplasia
Female
Fetal Growth Retardation
Humans
Hypertension, Pregnancy-Induced
Hypotension
Infant
Infant, Newborn*
Infant, Premature
Logistic Models
Mortality
Nitric Oxide
Obstetric Labor, Premature
Odds Ratio
Outcome Assessment (Health Care)
Parturition
Pregnancy
Prenatal Care
Retrospective Studies
Seoul
Steroids
Adrenal Cortex Hormones
Nitric Oxide
Steroids
Figure
Reference
-
1. Committee on Obstetric Practice. Committee opinion no. 713: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017; 130:e102–9.2. Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972; 50:515–25.3. Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017; 3:CD004454.4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994; 12:1–24.5. Magann EF, Haram K, Ounpraseuth S, Mortensen JH, Spencer HJ, Morrison JC. Use of antenatal corticosteroids in special circumstances: a comprehensive review. Acta Obstet Gynecol Scand. 2017; 96:395–409.6. Ishikawa H, Miyazaki K, Ikeda T, Murabayashi N, Hayashi K, Kai A, et al. The effects of antenatal corticosteroids on short- and long-term outcomes in small-for-gestational-age infants. Int J Med Sci. 2015; 12:295–300.7. Melamed N, Pittini A, Barrett J, Shah J, Yoon EW, Lemyre B, et al. Antenatal corticosteroids and outcomes of small-for-gestational-age neonates. Obstet Gynecol. 2016; 128:1001–8.8. Kim WJ, Han YS, Ko HS, Park IY, Shin JC, Wie JH. Antenatal corticosteroids and outcomes of preterm small-for-gestationalage neonates in a single medical center. Obstet Gynecol Sci. 2018; 61:7–13.9. Torrance HL, Mulder EJ, Brouwers HA, van Bel F, Visser GH. Respiratory outcome in preterm small for gestational age fetuses with or without abnormal umbilical artery Doppler and/or maternal hypertension. J Matern Fetal Neonatal Med. 2007; 20:613–21.10. Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol. 2000; 182(1 Pt 1):198–206.11. van Stralen G, van der Bos J, Lopriore E, Te Pas AB, Bloemenkamp KW, Walther FJ, et al. No short-term benefits of antenatal corticosteroid treatment in severely preterm growth restricted fetuses: a case-control study. Early Hum Dev. 2009; 85:253–7.12. Schaap AH, Wolf H, Bruinse HW, Smolders-De Haas H, Van Ertbruggen I, Treffers PE. Effects of antenatal corticosteroid administration on mortality and long-term morbidity in early preterm, growth-restricted infants. Obstet Gynecol. 2001; 97:954–60.13. Simchen MJ, Alkazaleh F, Adamson SL, Windrim R, Telford J, Beyene J, et al. The fetal cardiovascular response to antenatal steroids in severe early-onset intrauterine growth restriction. Am J Obstet Gynecol. 2004; 190:296–304.14. Sharma D, Shastri S, Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin Med Insights Pediatr. 2016; 10:67–83.15. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978; 187:1–7.16. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92:529–34.17. Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010; 125:e214–24.18. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants: 2013 update. Neonatology. 2013; 103:353–68.19. Economides DL, Nicolaides KH, Linton EA, Perry LA, Chard T. Plasma cortisol and adrenocorticotropin in appropriate and small for gestational age fetuses. Fetal Ther. 1988; 3:158–64.20. Torrance HL, Derks JB, Scherjon SA, Wijnberger LD, Visser GH. Is antenatal steroid treatment effective in preterm IUGR fetuses? Acta Obstet Gynecol Scand. 2009; 88:1068–73.21. Miller SL, Chai M, Loose J, Castillo-Melendez M, Walker DW, Jenkin G, et al. The effects of maternal betamethasone administration on the intrauterine growth-restricted fetus. Endocrinology. 2007; 148:1288–95.22. McTernan CL, Draper N, Nicholson H, Chalder SM, Driver P, Hewison M, et al. Reduced placental 11beta-hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms. J Clin Endocrinol Metab. 2001; 86:4979–83.23. Ozdemir H, Guvenal T, Cetin M, Kaya T, Cetin A. A placebocontrolled comparison of effects of repetitive doses of betamethasone and dexamethasone on lung maturation and lung, liver, and body weights of mouse pups. Pediatr Res. 2003; 53:98–103.24. Bertram C, Trowern AR, Copin N, Jackson AA, Whorwood CB. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11betahydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology. 2001; 142:2841–53.25. Morrison JL, Orgeig S. Antenatal glucocorticoid treatment of the growth-restricted fetus: benefit or cost? Reprod Sci. 2009; 16:527–38.26. Riskin-Mashiah S, Riskin A, Bader D, Kugelman A, Boyko V, Lerner-Geva L, et al. Antenatal corticosteroid treatment in singleton, small-for-gestational-age infants born at 24-31 weeks' gestation: a population-based study. BJOG. 2016; 123:1779–86.27. Ting JY, Kingdom JC, Shah PS. Antenatal glucocorticoids, magnesium sulfate, and mode of birth in preterm fetal small for gestational age. Am J Obstet Gynecol. 2018; 218(2S):S818–28.28. McGillick EV, Orgeig S, Williams MT, Morrison JL. Risk of respiratory distress syndrome and efficacy of glucocorticoids: are they the same in the normally grown and growth-restricted infant? Reprod Sci. 2016; 23:1459–72.29. Elimian A, Figueroa R, Spitzer AR, Ogburn PL, Wiencek V, Quirk JG. Antenatal corticosteroids: are incomplete courses beneficial? Obstet Gynecol. 2003; 102:352–5.30. Miller SL, Supramaniam VG, Jenkin G, Walker DW, Wallace EM. Cardiovascular responses to maternal betamethasone administration in the intrauterine growth-restricted ovine fetus. Am J Obstet Gynecol. 2009; 201:613. e1-8.31. Lai MY, Chu SM, Lakshminrusimha S, Lin HC. Beyond the inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Pediatr Neonatol. 2018; 59:15–23.32. Mitsiakos G, Kovacs L, Papageorgiou A. Are antenatal steroids beneficial to severely growth restricted fetuses? J Matern Fetal Neonatal Med. 2013; 26:1496–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of antenatal corticosteroid therapy on fetal growth
- The Effect of Incompletely Administered Antenatal Corticosteroids on Neonatal Pulmonary Outcomes in Late Preterm Infants
- The effect of time interval between a single course of antenatal corticosteroids and delivery on outcomes in preterm neonates
- The Benefits and Risks of Multiple Courses of Antenatal Corticosteroid Therapy in Patients with Preterm Premature Rupture of Membranes
- The Effect of Antenatal Corticosteroid on Perinatal Outcomes of Preterm Births