Neonatal Med.
2018 Aug;25(3):109-117. 10.5385/nm.2018.25.3.109.
Clinical Outcomes of Minimally Invasive Surfactant Therapy via Tracheal Catheterization in Neonates with a Gestational Age of 30 Weeks or More Diagnosed with Respiratory Distress Syndrome
- Affiliations
-
- 1Department of Pediatrics, Inje University Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea. peddoc@paik.ac.kr
- KMID: 2436127
- DOI: http://doi.org/10.5385/nm.2018.25.3.109
Abstract
- PURPOSE
Minimally invasive surfactant therapy (MIST) is currently used as a method of surfactant replacement therapy (SRT) for the treatment of respiratory distress syndrome (RDS) in preterm infants with a gestational age of less than 30 weeks. However, few studies have been conducted on MIST in neonates with a gestational age of 30 weeks or more. In this study, we compared MIST with endotracheal intubation as a rescue SRT for spontaneously breathing neonates with a gestational age of 30 weeks or more who were diagnosed with RDS.
METHODS
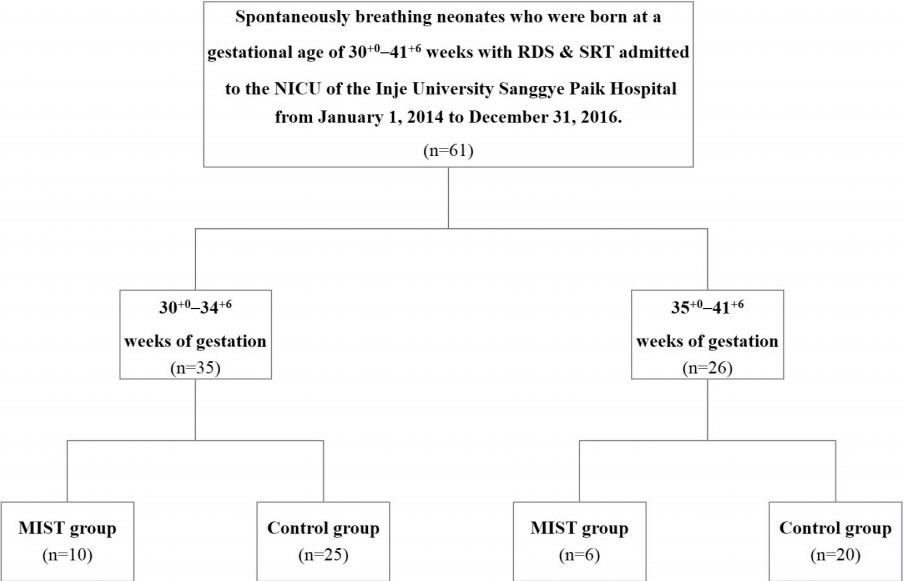
We investigated the clinical characteristics of spontaneously breathing neonates admitted to the neonatal intensive care unit of the Inje University Sanggye Paik Hospital from January 1, 2014 to December 31, 2016. These neonates were born at a gestational age of 30 weeks or more and were diagnosed with RDS. The neonates who were administered surfactant by MIST were categorized into the MIST group (n=16) and those who underwent endotracheal intubation were categorized into the control group (n=45). Thereafter, the clinical characteristics between the groups were compared.
RESULTS
Compared to the control group, the MIST group was less likely to require mechanical ventilation within 72 hours (P < 0.001). The frequency of bradycardia during SRT was also low in the MIST group (P=0.033).
CONCLUSION
MIST is considered relatively feasible and safe for treating RDS for reducing the need for mechanical ventilation and decreasing the occurrence of bradycardia during surfactant administration in neonates with a gestational age of 30 weeks or more.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
History of Pulmonary Surfactant Replacement Therapy for Neonatal Respiratory Distress Syndrome in Korea
Chong-Woo Bae, Chae Young Kim, Sung-Hoon Chung, Yong-Sung Choi
J Korean Med Sci. 2019;34(25):. doi: 10.3346/jkms.2019.34.e175.
Reference
-
1. Speer CP. Neonatal respiratory distress syndrome: an inflammatory disease? Neonatology. 2011; 99:316–9.2. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of respiratory distress syndrome: 2016 update. Neonatology. 2017; 111:107–25.3. Committee on Fetus and Newborn; American Academy of Pediatrics. Respiratory support in preterm infants at birth. Pediatrics. 2014; 133:171–4.4. Verder H, Robertson B, Greisen G, Ebbesen F, Albertsen P, Lundstrom K, et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N Engl J Med. 1994; 331:1051–5.5. Verder H, Albertsen P, Ebbesen F, Greisen G, Robertson B, Bertelsen A, et al. Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks' gestation. Pediatrics. 1999; 103:E24.6. Venkatesh V, Ponnusamy V, Anandaraj J, Chaudhary R, Malviya M, Clarke P, et al. Endotracheal intubation in a neonatal population remains associated with a high risk of adverse events. Eur J Pediatr. 2011; 170:223–7.7. Nilsson R, Grossmann G, Robertson B. Bronchiolar epithelial lesions induced in the premature rabbit neonate by short periods of artificial ventilation. Acta Pathol Microbiol Scand A. 1980; 88:359–67.8. Kolatat T, Aunganon K, Yosthiem P. Airway complications in neonates who received mechanical ventilation. J Med Assoc Thai. 2002; 85 Suppl 2:S455–62.9. Allen KA. Premedication for neonatal intubation: which medications are recommended and why. Adv Neonatal Care. 2012; 12:107–11.10. Shim GH. Update of minimally invasive surfactant therapy. Korean J Pediatr. 2017; 60:273–81.11. Gopel W, Kribs A, Ziegler A, Laux R, Hoehn T, Wieg C, et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an openlabel, randomised, controlled trial. Lancet. 2011; 378:1627–34.12. Gortner L, Bartmann P, Pohlandt F, Bernsau U, Porz F, Hellwege HH, et al. Early treatment of respiratory distress syndrome with bovine surfactant in very preterm infants: a multicenter controlled clinical trial. Pediatr Pulmonol. 1992; 14:4–9.13. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001; 163:1723–9.14. Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987; 17:213–88.15. International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005; 123:991–9.16. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92:529–34.17. Aldana-Aguirre JC, Pinto M, Featherstone RM, Kumar M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2017; 102:F17–23.18. Lopez E, Gascoin G, Flamant C, Merhi M, Tourneux P, Baud O, et al. Exogenous surfactant therapy in 2013: what is next? Who, when and how should we treat newborn infants in the future? BMC Pediatr. 2013; 13:165.19. Bohlin K, Bouhafs RK, Jarstrand C, Curstedt T, Blennow M, Robertson B. Spontaneous breathing or mechanical ventilation alters lung compliance and tissue association of exogenous surfactant in preterm newborn rabbits. Pediatr Res. 2005; 57:624–30.20. Klebermass-Schrehof K, Wald M, Schwindt J, Grill A, Prusa AR, Haiden N, et al. Less invasive surfactant administration in extremely preterm infants: impact on mortality and morbidity. Neonatology. 2013; 103:252–8.21. Aguar M, Cernada M, Brugada M, Gimeno A, Gutierrez A, Vento M. Minimally invasive surfactant therapy with a gastric tube is as effective as the intubation, surfactant, and extubation technique in preterm babies. Acta Paediatr. 2014; 103:e229. –33.22. Tomar RS, Ghuliani R, Yadav D. Effect of surfactant therapy using orogastric tube for tracheal catheterization in preterm newborns with respiratory distress. Indian J Pediatr. 2017; 84:257–61.23. Jones P, Dauger S, Peters MJ. Bradycardia during critical care intubation: mechanisms, significance and atropine. Arch Dis Child. 2012; 97:139–44.24. Kumar P, Denson SE, Mancuso TJ; Committee on Fetus and Newborn. Section on Anesthesiology and Pain Medicine. Premedication for nonemergency endotracheal intubation in the neonate. Pediatrics. 2010; 125:608–15.25. Klotz D, Porcaro U, Fleck T, Fuchs H. European perspective on less invasive surfactant administration-a survey. Eur J Pediatr. 2017; 176:147–54.26. Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U. Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics. 2013; 131:e502. –9.27. Dargaville PA, Ali SKM, Jackson HD, Williams C, De Paoli AG. Impact of minimally invasive surfactant therapy in preterm infants at 29-32 weeks gestation. Neonatology. 2018; 113:7–14.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Minimally Invasive Surfactant Therapy
- Comparison of minimally invasive surfactant therapy with intubation surfactant administration and extubation for treating preterm infants with respiratory distress syndrome: a randomized clinical trial
- Surfactant Therapy in Respiratory Distress Syndrome
- Sequential Changes of Chest Radiographic Finding after Exogenous Surfactant Replacement Therapy in Neonates with RDS
- Comparison of Respiratory Outcome between the Surfactant without Endotracheal Tube Intubation and the Intubation-SurfactantExtubation Techniques in Extremely Low Gestational Age Neonates with Respiratory Distress Syndrome