Investig Clin Urol.
2019 Jan;60(1):4-13. 10.4111/icu.2019.60.1.4.
Magnetic resonance imaging-transrectal ultrasound fusion image-guided prostate biopsy: Current status of the cancer detection and the prospects of tailor-made medicine of the prostate cancer
- Affiliations
-
- 1Department of Urology, Tokai University Hachioji Hospital, Hachioji, Tokyo, Japan. sunashoj@mail.goo.ne.jp
- KMID: 2434619
- DOI: http://doi.org/10.4111/icu.2019.60.1.4
Abstract
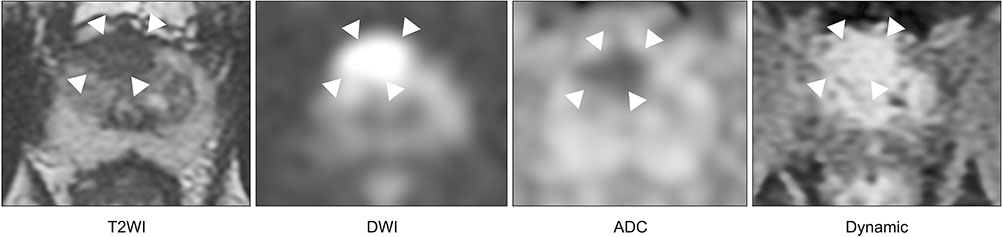
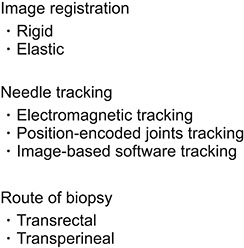
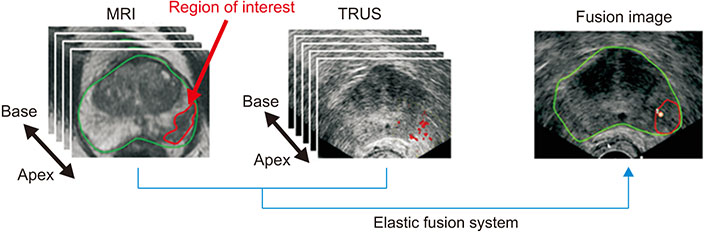
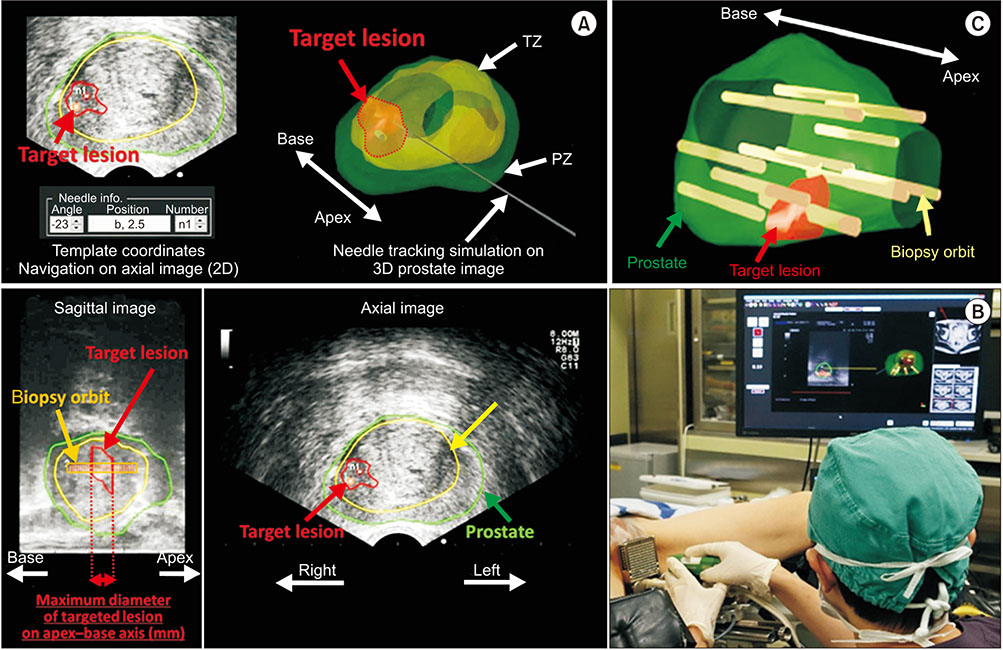
- Multi-parametric magnetic resonance imaging (mpMRI) has been increasingly used to diagnose clinically significant prostate cancer (csPCa) because of its growing availability and its ability to combine anatomical and functional data. Magnetic resonance imaging (MRI)-transrectal ultrasound (TRUS) fusion imaging provides MRI information with TRUS images for prostate biopsies. This technique combines the superior sensitivity of MRI for targeting suspicious lesions with the practicality and familiarity of TRUS. MRI-TRUS fusion image-guided prostate biopsy is performed with different types of image registration (rigid vs. elastic) and needle tracking methods (electromagnetic tracking vs. mechanical position encoders vs. image-based software tracking). A systematic review and meta-analysis showed that MRI-targeted biopsy detected csPCa at a significantly higher rate than did TRUS-guided biopsy, while it detected significantly fewer cases of insignificant PCas. In addition to the high accuracy of MRI-targeted biopsy for csPCa, localization of csPCa is accurate. The ability to choose the route of biopsy (transperineal vs. transrectal) is required, depending on the patients' risk and the location and size of suspicious lesions on mpMRI. Fusion image-guided prostate biopsy has the potential to allow precise management of prostate cancer, including active surveillance, radical treatment, and focal therapy.
MeSH Terms
Figure
Cited by 1 articles
-
Diagnostic Accuracy and Value of Magnetic Resonance Imaging–Ultrasound Fusion Transperineal Targeted and Template Systematic Prostate Biopsy Based on Bi-parametric Magnetic Resonance Imaging
Tae Il Noh, Jong Hyun Tae, Hyung Keun Kim, Ji Sung Shim, Sung Gu Kang, Deuk Jae Sung, Jun Cheon, Jeong Gu Lee, Seok Ho Kang
Cancer Res Treat. 2020;52(3):714-721. doi: 10.4143/crt.2019.716.
Reference
-
1. Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012; 22:746–757.
Article2. Toi A, Neill MG, Lockwood GA, Sweet JM, Tammsalu LA, Fleshner NE. The continuing importance of transrectal ultrasound identification of prostatic lesions. J Urol. 2007; 177:516–520.
Article3. Dickinson L, Ahmed HU, Allen C, Barentsz JO, Carey B, Futterer JJ, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol. 2011; 59:477–494.
Article4. Vilanova JC, Barceló-Vidal C, Comet J, Boada M, Barceló J, Ferrer J, et al. Usefulness of prebiopsy multifunctional and morphologic MRI combined with free-to-total prostate-specific antigen ratio in the detection of prostate cancer. AJR Am J Roentgenol. 2011; 196:W715–W722.
Article5. Delongchamps NB, Rouanne M, Flam T, Beuvon F, Liberatore M, Zerbib M, et al. Multiparametric magnetic resonance imaging for the detection and localization of prostate cancer: combination of T2-weighted, dynamic contrast-enhanced and diffusion-weighted imaging. BJU Int. 2011; 107:1411–1418.
Article6. Mottet N, Clarke N, De Santis M, Zattoni F, Morote J, Joniau S. Implementing newer agents for the management of castrate-resistant prostate cancer: what is known and what is needed? BJU Int. 2015; 115:364–372.
Article7. Moore CM, Kasivisvanathan V, Eggener S, Emberton M, Fütterer JJ, Gill IS, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol. 2013; 64:544–552.
Article8. Sciarra A, Barentsz J, Bjartell A, Eastham J, Hricak H, Panebianco V, et al. Advances in magnetic resonance imaging: how they are changing the management of prostate cancer. Eur Urol. 2011; 59:962–977.
Article9. Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA, Hulsbergen-van de Kaa CA, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011; 259:453–461.
Article10. Kasivisvanathan V, Dufour R, Moore CM, Ahmed HU, Abd-Alazeez M, Charman SC, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol. 2013; 189:860–866.
Article11. Hambrock T, Somford DM, Hoeks C, Bouwense SA, Huisman H, Yakar D, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010; 183:520–527.
Article12. Wegelin O, van Melick HHE, Hooft L, Bosch JLHR, Reitsma HB5, Barentsz JO, et al. Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: a systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique? Eur Urol. 2017; 71:517–531.
Article13. Haffner J, Lemaitre L, Puech P, Haber GP, Leroy X, Jones JS, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011; 108:E171–E178.
Article14. Park BK, Park JW, Park SY, Kim CK, Lee HM, Jeon SS, et al. Prospective evaluation of 3-T MRI performed before initial transrectal ultrasound-guided prostate biopsy in patients with high prostate-specific antigen and no previous biopsy. AJR Am J Roentgenol. 2011; 197:W876–W881.
Article15. Sciarra A, Panebianco V, Ciccariello M, Salciccia S, Cattarino S, Lisi D, et al. Value of magnetic resonance spectroscopy imaging and dynamic contrast-enhanced imaging for detecting prostate cancer foci in men with prior negative biopsy. Clin Cancer Res. 2010; 16:1875–1883.
Article16. Labanaris AP, Engelhard K, Zugor V, Nützel R, Kühn R. Prostate cancer detection using an extended prostate biopsy schema in combination with additional targeted cores from suspicious images in conventional and functional endorectal magnetic resonance imaging of the prostate. Prostate Cancer Prostatic Dis. 2010; 13:65–70.
Article17. Sonn GA, Margolis DJ, Marks LS. Target detection: magnetic resonance imaging-ultrasound fusion-guided prostate biopsy. Urol Oncol. 2014; 32:903–911.
Article18. Moore CM, Robertson NL, Arsanious N, Middleton T, Villers A, Klotz L, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol. 2013; 63:125–140.
Article19. Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging - reporting and data system: 2015, version 2. Eur Urol. 2016; 69:16–40.
Article20. Barret E, Turkbey B, Puech P, Durand M, Panebianco V, Fütterer JJ, et al. Update on the ICUD-SIU consultation on multi-parametric magnetic resonance imaging in localised prostate cancer. World J Urol. 2018; 07. 12. [Epub]. DOI: 10.1007/S00345-018-2395-3.
Article21. Collins DJ, Padhani AR. Dynamic magnetic resonance imaging of tumor perfusion. Approaches and biomedical challenges. IEEE Eng Med Biol Mag. 2004; 23:65–83.22. Hara N, Okuizumi M, Koike H, Kawaguchi M, Bilim V. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is a useful modality for the precise detection and staging of early prostate cancer. Prostate. 2005; 62:140–147.
Article23. Gibbs P, Liney GP, Pickles MD, Zelhof B, Rodrigues G, Turnbull LW. Correlation of ADC and T2 measurements with cell density in prostate cancer at 3.0 Tesla. Invest Radiol. 2009; 44:572–576.
Article24. van As NJ, de Souza NM, Riches SF, Morgan VA, Sohaib SA, Dearnaley DP, et al. A study of diffusion-weighted magnetic resonance imaging in men with untreated localised prostate cancer on active surveillance. Eur Urol. 2009; 56:981–987.
Article25. Zelhof B, Pickles M, Liney G, Gibbs P, Rodrigues G, Kraus S, et al. Correlation of diffusion-weighted magnetic resonance data with cellularity in prostate cancer. BJU Int. 2009; 103:883–888.
Article26. Turkbey B, Shah VP, Pang Y, Bernardo M, Xu S, Kruecker J, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011; 258:488–495.
Article27. Oto A, Kayhan A, Jiang Y, Tretiakova M, Yang C, Antic T, et al. Prostate cancer: differentiation of central gland cancer from benign prostatic hyperplasia by using diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology. 2010; 257:715–723.
Article28. Rud E, Klotz D, Rennesund K, Baco E, Berge V, Lien D, et al. Detection of the index tumour and tumour volume in prostate cancer using T2-weighted and diffusion-weighted magnetic resonance imaging (MRI) alone. BJU Int. 2014; 114:E32–E42.
Article29. Baco E, Ukimura O, Rud E, Vlatkovic L, Svindland A, Aron M, et al. Magnetic resonance imaging-transectal ultrasound image-fusion biopsies accurately characterize the index tumor: correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur Urol. 2015; 67:787–794.
Article30. Rosenkrantz AB, Mendrinos S, Babb JS, Taneja SS. Prostate cancer foci detected on multiparametric magnetic resonance imaging are histologically distinct from those not detected. J Urol. 2012; 187:2032–2038.
Article31. Hamoen EHJ, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: a diagnostic meta-analysis. Eur Urol. 2015; 67:1112–1121.
Article32. Junker D, Quentin M, Nagele U, Edlinger M, Richenberg J, Schaefer G, et al. Evaluation of the PI-RADS scoring system for mpMRI of the prostate: a whole-mount step-section analysis. World J Urol. 2015; 33:1023–1030.
Article33. Steiger P, Thoeny HC. Prostate MRI based on PI-RADS version 2: how we review and report. Cancer Imaging. 2016; 16:9.
Article34. Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol. 2015; 68:438–450.
Article35. Delongchamps NB, Peyromaure M, Schull A, Beuvon F, Bouazza N, Flam T, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol. 2013; 189:493–499.
Article36. Rud E, Baco E, Eggesbø HB. MRI and ultrasound-guided prostate biopsy using soft image fusion. Anticancer Res. 2012; 32:3383–3389.37. Logan JK, Rais-Bahrami S, Turkbey B, Gomella A, Amalou H, Choyke PL, et al. Current status of magnetic resonance imaging (MRI) and ultrasonography fusion software platforms for guidance of prostate biopsies. BJU Int. 2014; 114:641–652.
Article38. Shoji S, Hiraiwa S, Endo J, Hashida K, Tomonaga T, Nakano M, et al. Manually controlled targeted prostate biopsy with real-time fusion imaging of multiparametric magnetic resonance imaging and transrectal ultrasound: an early experience. Int J Urol. 2015; 22:173–178.
Article39. Shoji S, Hiraiwa S, Ogawa T, Kawakami M, Nakano M, Hashida K, et al. Accuracy of real-time magnetic resonance imaging-transrectal ultrasound fusion image-guided transperineal target biopsy with needle tracking with a mechanical position-encoded stepper in detecting significant prostate cancer in biopsy-naïve men. Int J Urol. 2017; 24:288–294.
Article40. Radtke JP, Schwab C, Wolf MB, Freitag MT, Alt CD, Kesch C, et al. Multiparametric magnetic resonance imaging (MRI) and MRI-transrectal ultrasound fusion biopsy for index tumor detection: correlation with radical prostatectomy specimen. Eur Urol. 2016; 70:846–853.
Article41. Wysock JS, Rosenkrantz AB, Huang WC, Stifelman MD, Lepor H, Deng FM, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol. 2014; 66:343–351.
Article42. Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015; 313:390–397.
Article43. Oberlin DT, Casalino DD, Miller FH, Matulewicz RS, Perry KT, Nadler RB, et al. Diagnostic value of guided biopsies: fusion and cognitive-registration magnetic resonance imaging versus conventional ultrasound biopsy of the prostate. Urology. 2016; 92:75–79.
Article44. Kongnyuy M, George AK, Rastinehad AR, Pinto PA. Magnetic resonance imaging-ultrasound fusion-guided prostate biopsy: review of technology, techniques, and outcomes. Curr Urol Rep. 2016; 17:32.
Article45. Mozer P, Rouprêt M, Le Cossec C, Granger B, Comperat E, de Gorski A, et al. First round of targeted biopsies using magnetic resonance imaging/ultrasonography fusion compared with conventional transrectal ultrasonography-guided biopsies for the diagnosis of localised prostate cancer. BJU Int. 2015; 115:50–57.
Article46. Hara R, Jo Y, Fujii T, Kondo N, Yokoyoma T, Miyaji Y, et al. Optimal approach for prostate cancer detection as initial biopsy: prospective randomized study comparing transperineal versus transrectal systematic 12-core biopsy. Urology. 2008; 71:191–195.
Article47. Takenaka A, Hara R, Ishimura T, Fujii T, Jo Y, Nagai A, et al. A prospective randomized comparison of diagnostic efficacy between transperineal and transrectal 12-core prostate biopsy. Prostate Cancer Prostatic Dis. 2008; 11:134–138.
Article48. Huang H, Wang W, Lin T, Zhang Q, Zhao X, Lian H, et al. Comparison of the complications of traditional 12 cores transrectal prostate biopsy with image fusion guided transperineal prostate biopsy. BMC Urol. 2016; 16:68.
Article49. Steensels D, Slabbaert K, De Wever L, Vermeersch P, Van Poppel H, Verhaegen J. Fluoroquinolone-resistant E. coli in intestinal flora of patients undergoing transrectal ultrasound-guided prostate biopsy-should we reassess our practices for antibiotic prophylaxis? Clin Microbiol Infect. 2012; 18:575–581.
Article50. Guo LH, Wu R, Xu HX, Xu JM, Wu J, Wang S, et al. Comparison between ultrasound guided transperineal and transrectal prostate biopsy: a prospective, randomized, and controlled trial. Sci Rep. 2015; 11. 03. [Epub]. DOI: 10.1038/srep16089.
Article51. Smith JB, Popert R, Nuttall MC, Vyas L, Kinsella J, Cahill D. Transperineal sector prostate biopsies: a local anesthetic outpatient technique. Urology. 2014; 83:1344–1349.52. Tewes S, Peters I, Tiemeyer A, Peperhove M1, Hartung D1, Pertschy S, et al. Evaluation of MRI/ultrasound fusion-guided prostate biopsy using transrectal and transperineal approaches. Biomed Res Int. 2017; 2017:2176471.
Article53. Venderink W, de Rooij M, Sedelaar JPM, Huisman HJ, Fütterer JJ. Elastic versus rigid image registration in magnetic resonance imaging-transrectal ultrasound fusion prostate biopsy: a systematic review and meta-analysis. Eur Urol Focus. 2018; 4:219–227.
Article54. Mohler JL. NCCN Prostate Cancer Panel. Joint statement by members of the NCCN Prostate Cancer Guidelines Panel. J Natl Compr Canc Netw. 2013; 11:1310–1312.
Article55. van den Bergh RC, Roemeling S, Roobol MJ, Aus G, Hugosson J, Rannikko AS, et al. Outcomes of men with screen-detected prostate cancer eligible for active surveillance who were managed expectantly. Eur Urol. 2009; 55:1–8.
Article56. Porten SP, Whitson JM, Cowan JE, Perez N, Shinohara K, Carroll PR. Changes in cancer volume in serial biopsies of men on active surveillance for early stage prostate cancer. J Urol. 2011; 186:1825–1829.
Article57. Shoji S, Ukimura O, de Castro Abreu AL, Marien A, Matsugasumi T, Bahn D, et al. Image-based monitoring of targeted biopsy-proven prostate cancer on active surveillance: 11-year experience. World J Urol. 2016; 34:221–227.
Article58. Stavrinides V, Giganti F, Emberton M, Moore CM. MRI in active surveillance: a critical review. Prostate Cancer Prostatic Dis. 2018; 08. 16. [Epub]. DOI: 10.1038/s41391-018-0077-2.
Article59. Hung AJ, Abreu AL, Shoji S, Goh AC, Berger AK, Desai MM, et al. Robotic transrectal ultrasonography during robot-assisted radical prostatectomy. Eur Urol. 2012; 62:341–348.
Article60. Humphrey PA. Complete histologic serial sectioning of a prostate gland with adenocarcinoma. Am J Surg Pathol. 1993; 17:468–472.
Article61. Gangi A, Tsoumakidou G, Abdelli O, Buy X, de Mathelin M, Jacqmin D, et al. Percutaneous MR-guided cryoablation of prostate cancer: initial experience. Eur Radiol. 2012; 22:1829–1835.
Article62. Ahmed HU, Hindley RG, Dickinson L, Freeman A, Kirkham AP, Sahu M, et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol. 2012; 13:622–632.
Article63. Muto S, Yoshii T, Saito K, Kamiyama Y, Ide H, Horie S. Focal therapy with high-intensity-focused ultrasound in the treatment of localized prostate cancer. Jpn J Clin Oncol. 2008; 38:192–199.
Article64. Ahmed HU, Freeman A, Kirkham A, Sahu M, Scott R, Allen C, et al. Focal therapy for localized prostate cancer: a phase I/II trial. J Urol. 2011; 185:1246–1254.
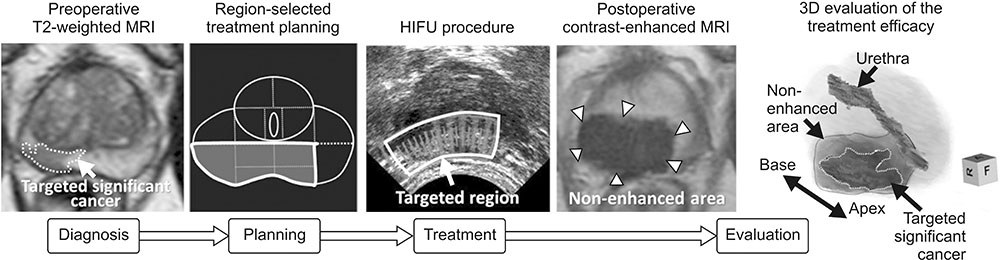
Article65. Shoji S, Mouraviev V, Scionti S. High intensity focused ultrasound (HIFU) treatment of prostate cancer. In : Johansen TEB, Greene D, Breen DJ, Mouraviev V, editors. Handbook of Focal Therapy for Prostate and Renal Cancer. London: JP Medical Ltd;2016. p. 241–254.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medical imaging of prostate cancer
- Pain during Transrectal Ultrasound-Guided Prostate Biopsy and the Role of Periprostatic Nerve Block: What Radiologists Should Know
- Magnetic Resonance Imaging-Guided Prostate Biopsy: Present and Future
- Multiparametric MRI in the Detection of Clinically Significant Prostate Cancer
- Added values of transrectal ultrasonography to magnetic resonance imaging in characterizing prostate cancer: A narrative review