Investig Magn Reson Imaging.
2018 Dec;22(4):209-217. 10.13104/imri.2018.22.4.209.
Susceptibility Weighted Imaging of the Cervical Spinal Cord with Compensation of Respiratory-Induced Artifact
- Affiliations
-
- 1Department of Electrical and Electronic Engineering, Yonsei University, Seoul, Korea. donghyunkim@yonsei.ac.kr
- 2Department of Radiology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Radiology, Gachon University Gil Medical Center, Incheon, Korea.
- KMID: 2431105
- DOI: http://doi.org/10.13104/imri.2018.22.4.209
Abstract
- PURPOSE
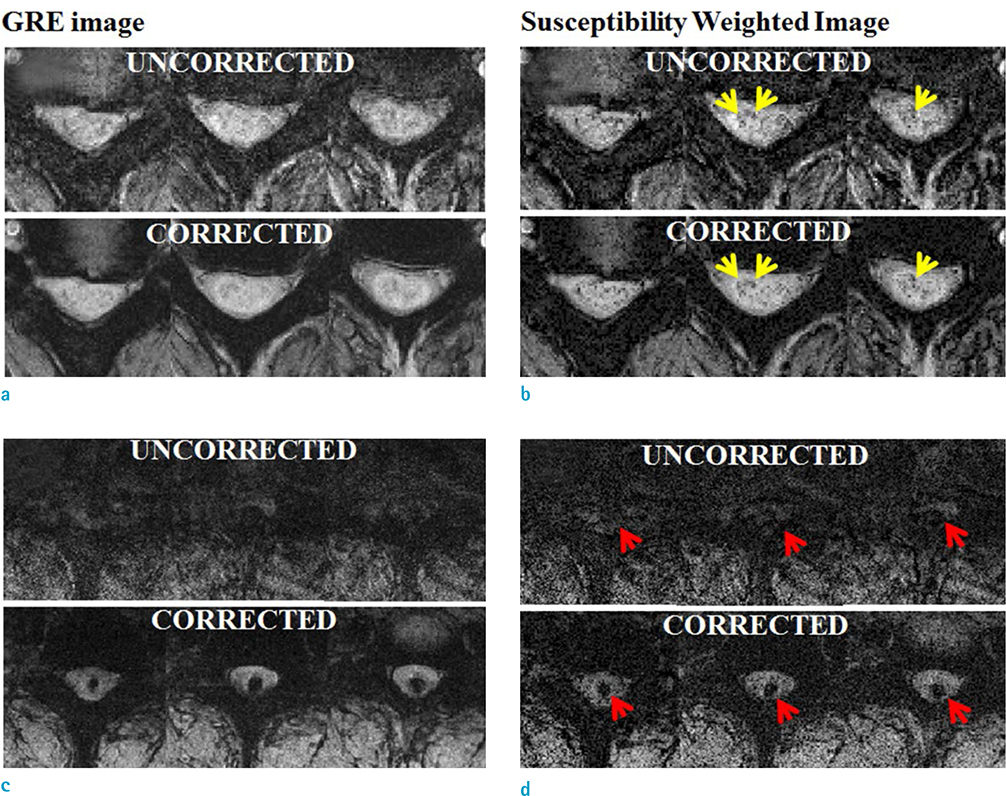
The objective of this study was to obtain improved susceptibility weighted images (SWI) of the cervical spinal cord using respiratory-induced artifact compensation.
MATERIALS AND METHODS
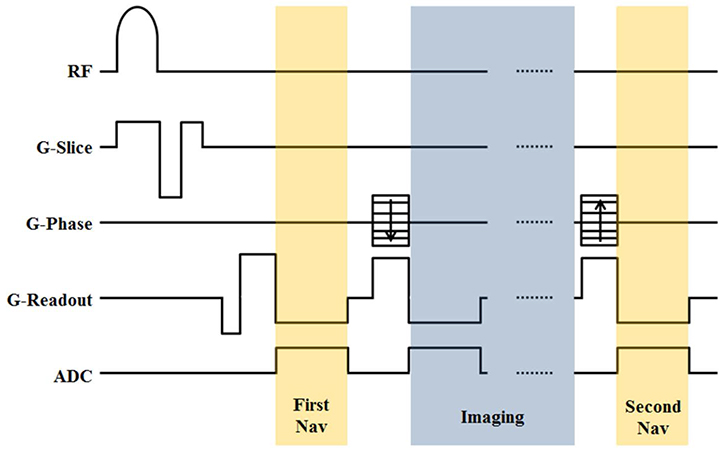
The artifact from B0 fluctuations by respiration could be compensated using a double navigator echo approach. The two navigators were inserted in an SWI sequence before and after the image readouts. The B0 fluctuation was measured by each navigator echoes, and the inverse of the fluctuation was applied to eliminate the artifact from fluctuation. The degree of compensation was quantified using a quality index (QI) term for compensated imaging using each navigator. Also, the effect of compensation was analyzed according to the position of the spinal cord using QI values.
RESULTS
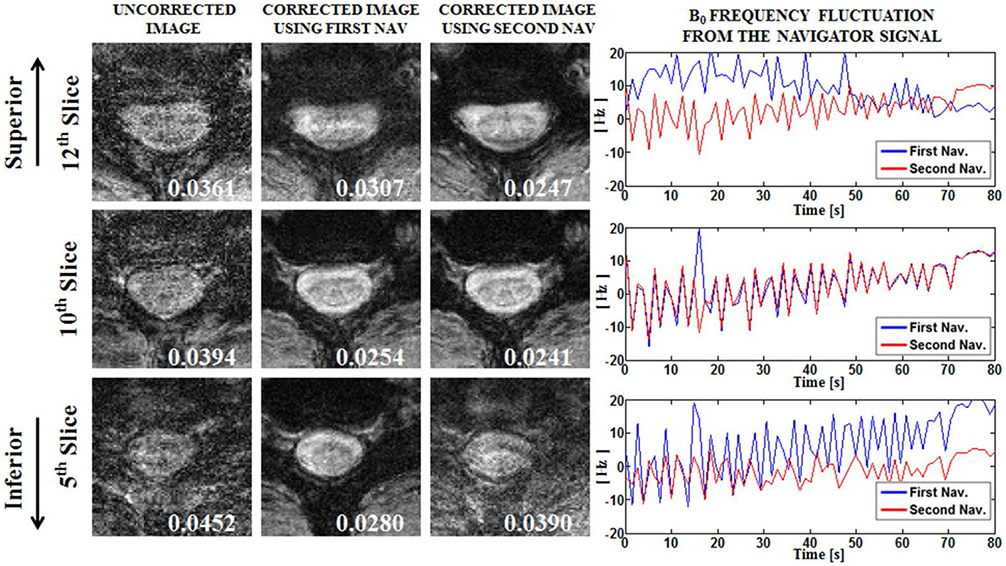
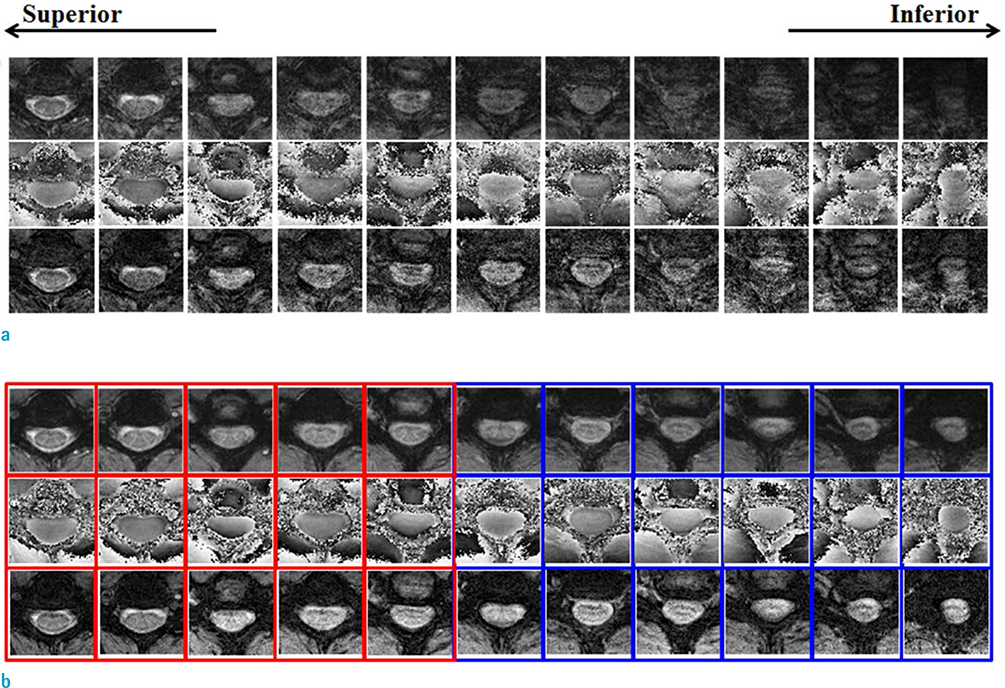
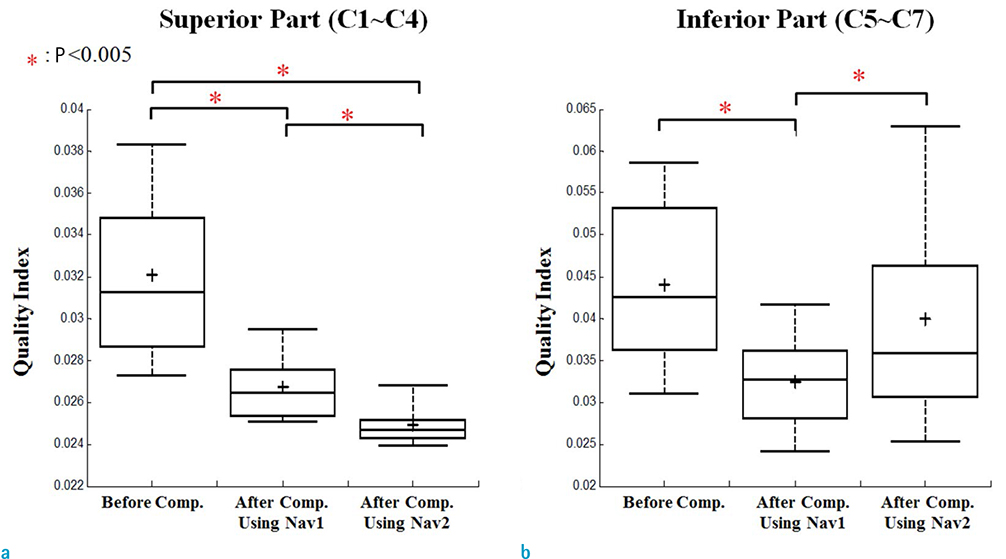
Compensation using navigator echo gave the improved visualization of SWI in cervical spinal cord compared to non-compensated images. Before compensation, images were influenced by artificial noise from motion in both the superior (QI = 0.031) and inferior (QI = 0.043) regions. In most parts of the superior regions, the second navigator resulted in better quality (QI = 0.024, P < 0.01) compared to the first navigator, but in the inferior regions the first navigator showed better quality (QI = 0.033, P < 0.01) after correction.
CONCLUSION
Motion compensation using a double navigator method can increase the improvement of the SWI in the cervical spinal cord. The proposed method makes SWI a useful tool for the diagnosis of spinal cord injury by reducing respiratory-induced artifact.
Keyword
MeSH Terms
Figure
Reference
-
1. Thomas B, Somasundaram S, Thamburaj K, et al. Clinical applications of susceptibility weighted MR imaging of the brain - a pictorial review. Neuroradiology. 2008; 50:105–116.
Article2. Wycliffe ND, Choe J, Holshouser B, Oyoyo UE, Haacke EM, Kido DK. Reliability in detection of hemorrhage in acute stroke by a new three-dimensional gradient recalled echo susceptibility-weighted imaging technique compared to computed tomography: a retrospective study. J Magn Reson Imaging. 2004; 20:372–377.
Article3. Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med. 2004; 52:612–618.
Article4. Wang M, Dai Y, Han Y, Haacke EM, Dai J, Shi D. Susceptibility weighted imaging in detecting hemorrhage in acute cervical spinal cord injury. Magn Reson Imaging. 2011; 29:365–373.
Article5. Mittal S, Wu Z, Neelavalli J, Haacke EM. Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol. 2009; 30:232–252.
Article6. Ishizaka K, Kudo K, Fujima N, et al. Detection of normal spinal veins by using susceptibility-weighted imaging. J Magn Reson Imaging. 2010; 31:32–38.
Article7. Biber S, Wohlfarth K, Kirsch J, Schmidt A. Design of a local shim coil to improve B0 homogeneity in the cervical spine region. In : Proceedings of the 20th Annual Meeting of ISMRM; Melbourne, Australia. 2012. p. 2746.8. Hock A, Fuchs A, Boesiger P, Kollias SS, Henning A. Electrocardiogram-triggered, higher order, projection-based B(0) shimming allows for fast and reproducible shim convergence in spinal cord (1)H MRS. NMR Biomed. 2013; 26:329–335.9. Stroman PW, Wheeler-Kingshott C, Bacon M, et al. The current state-of-the-art of spinal cord imaging: methods. Neuroimage. 2014; 84:1070–1081.
Article10. Verma T, Cohen-Adad J. Effect of respiration on the B0 field in the human spinal cord at 3T. Magn Reson Med. 2014; 72:1629–1636.11. Holland AE, Goldfarb JW, Edelman RR. Diaphragmatic and cardiac motion during suspended breathing: preliminary experience and implications for breath-hold MR imaging. Radiology. 1998; 209:483–489.
Article12. Ehman RL, McNamara MT, Pallack M, Hricak H, Higgins CB. Magnetic resonance imaging with respiratory gating: techniques and advantages. AJR Am J Roentgenol. 1984; 143:1175–1182.
Article13. Anderson AW, Gore JC. Analysis and correction of motion artifacts in diffusion weighted imaging. Magn Reson Med. 1994; 32:379–387.
Article14. Durand E, van de Moortele PF, Pachot-Clouard M, Le Bihan D. Artifact due to B(0) fluctuations in fMRI: correction using the k-space central line. Magn Reson Med. 2001; 46:198–201.15. Butts K, de Crespigny A, Pauly JM, Moseley M. Diffusion-weighted interleaved echo-planar imaging with a pair of orthogonal navigator echoes. Magn Reson Med. 1996; 35:763–770.
Article16. Song R, Tipirneni A, Johnson P, Loeffler RB, Hillenbrand CM. Evaluation of respiratory liver and kidney movements for MRI navigator gating. J Magn Reson Imaging. 2011; 33:143–148.
Article17. Wen J, Cross AH, Yablonskiy DA. On the role of physiological fluctuations in quantitative gradient echo MRI: implications for GEPCI, QSM, and SWI. Magn Reson Med. 2015; 73:195–203.
Article18. Fu ZW, Wang Y, Grimm RC, et al. Orbital navigator echoes for motion measurements in magnetic resonance imaging. Magn Reson Med. 1995; 34:746–753.
Article19. Nam Y, Kim DH, Lee J. Physiological noise compensation in gradient-echo myelin water imaging. Neuroimage. 2015; 120:345–349.
Article20. Lee J, Santos JM, Conolly SM, Miller KL, Hargreaves BA, Pauly JM. Respiration-induced B0 field fluctuation compensation in balanced SSFP: real-time approach for transition-band SSFP fMRI. Magn Reson Med. 2006; 55:1197–1201.
Article21. Lee H, Nam Y, Han D, Gho S-M, Kim D-H. SWI of the cervical-spinal cord with respiration noise correction using navigator echo. In : Proceedings of the 23rd Annual Meeting of ISMRM; Toronto, Ontario, Canada. 2015. p. 1727.22. Balu N, Chu B, Hatsukami TS, Yuan C, Yarnykh VL. Comparison between 2D and 3D high-resolution black-blood techniques for carotid artery wall imaging in clinically significant atherosclerosis. J Magn Reson Imaging. 2008; 27:918–924.
Article23. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002; 47:1202–1210.
Article24. Jang U, Nam Y, Kim DH, Hwang D. Improvement of the SNR and resolution of susceptibility-weighted venography by model-based multi-echo denoising. Neuroimage. 2013; 70:308–316.
Article25. Kaufman L, Kramer DM, Crooks LE, Ortendahl DA. Measuring signal-to-noise ratios in MR imaging. Radiology. 1989; 173:265–267.
Article26. McGee KP, Manduca A, Felmlee JP, Riederer SJ, Ehman RL. Image metric-based correction (autocorrection) of motion effects: analysis of image metrics. J Magn Reson Imaging. 2000; 11:174–181.
Article27. Mortamet B, Bernstein MA, Jack CR Jr, et al. Automatic quality assessment in structural brain magnetic resonance imaging. Magn Reson Med. 2009; 62:365–372.
Article28. Hu X, Kim SG. Reduction of signal fluctuation in functional MRI using navigator echoes. Magn Reson Med. 1994; 31:495–503.
Article29. Wowk B, McIntyre MC, Saunders JK. k-Space detection and correction of physiological artifacts in fMRI. Magn Reson Med. 1997; 38:1029–1034.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Motion Induced Artifact Mimicking Cervical Dens Fracture on the CT Scan: A Case Report
- Vertebral Artery Dissection Presenting with Acute Infarction in Cervical Spinal Cord and Cerebellum
- Morphological Analysis of the Cervical Spinal Cord, Dural Tube, and Spinal Canal by Magnetic Resonance Imaging in Normal Korean Adults
- Spinal Cord Injury by Ruptured Disc Particles in Cervical Spinal Trauma
- The “Lip Sign†in MRI of the Spinal Cord