J Gastric Cancer.
2018 Dec;18(4):348-355. 10.5230/jgc.2018.18.e34.
Adjuvant Chemotherapy with or without Concurrent Radiotherapy for Patients with Stage IB Gastric Cancer: a Subgroup Analysis of the Adjuvant Chemoradiotherapy in Stomach Tumors (ARTIST) Phase III Trial
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hematoma@skku.edu
- 2Division of Hematology-Oncology, Department of Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Republic of Korea.
- 3Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2429881
- DOI: http://doi.org/10.5230/jgc.2018.18.e34
Abstract
- PURPOSE
We aimed to discuss the roles of radiation and chemotherapy as adjuvant treatment in patients with staged IB GC who were enrolled in the adjuvant chemoradiotherapy in stomach tumors (ARTIST) trial.
MATERIALS AND METHODS
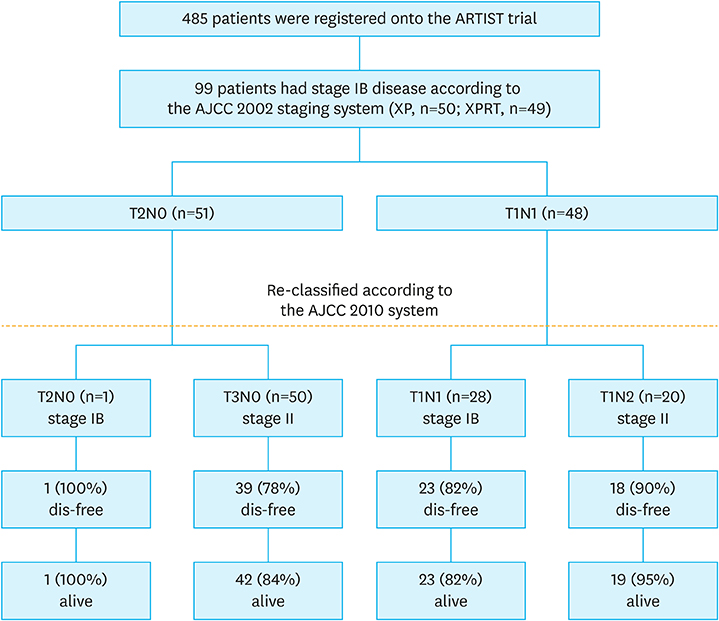
Among the 458 patients who were enrolled in the ARTIST trial, 99 had stage IB disease. The patients were randomly assigned to receive either adjuvant chemoradiotherapy with capecitabine plus cisplatin (XP, n=50) or chemoradiotherapy (XPRT, n=49). Survival analyses were performed in accordance with the AJCC 2010 staging system.
RESULTS
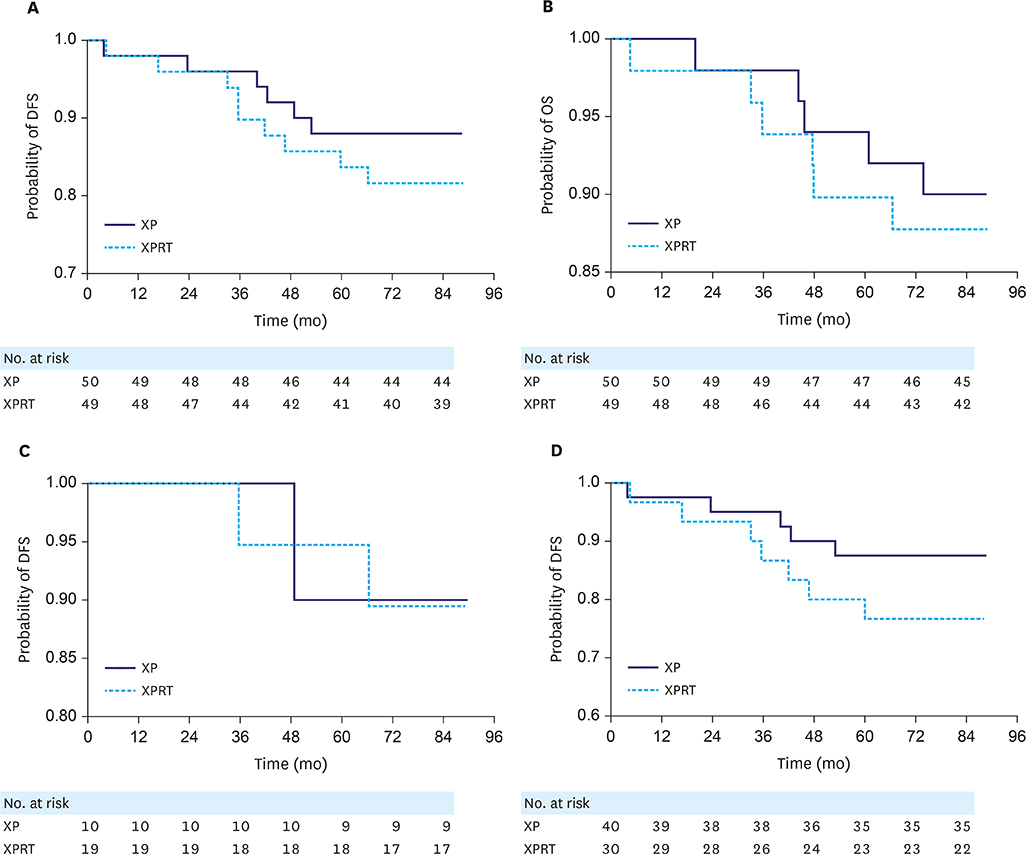
According to the AJCC 2010 system, stage migration from IB to II occurred in 71% of the patients; 98% of the T2 N0 cases were reclassified as T3 N0, and 42% of the T1 N1 cases were reclassified as T1 N2. When comparing survival outcomes between the XPRT and XP arms for stage IB cancer (AJCC 2002), no significant difference in 5-year disease-free survival (DFS) between the 2 arms was found. (median 5-year DFS, not reached, P=0.256). The patients classified as having stage IB cancer (AJCC 2002) and reclassified as having stage II cancer (AJCC 2010) exhibited worse prognoses than those who remained in stage IB, although the difference was not statistically significant (5-year DFS rate, 83% vs. 93%). When we compared 5-year DFS in 70 patients with stage II (AJCC 2010), the addition of radiotherapy to XP chemotherapy did not show better outcome than XP alone (P=0.137).
CONCLUSIONS
The role of adjuvant chemoradiotherapy in the treatment of stage IB GC (AJCC 2002) warrants further investigation.
MeSH Terms
Figure
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article2. Macdonald JS. Gastric cancer: Nagoya is not New York. J Clin Oncol. 2011; 29:4348–4350.
Article3. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001; 345:725–730.
Article4. Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999; 79:1522–1530.5. Bonenkamp JJ, Hermans J, Sasako M, van de Velde CJ, Welvaart K, Songun I, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999; 340:908–914.
Article6. Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, et al. Extended lymph node dissection for gastric cancer: who may benefit? final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004; 22:2069–2077.
Article7. Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008; 359:453–462.
Article8. Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012; 30:268–273.
Article9. Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol. 2015; 33:3130–3136.
Article10. Ajani JA, Bentrem DJ, Besh S, D'Amico TA, Das P, Denlinger C, et al. Gastric cancer, version 2.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013; 11:531–546.11. Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010; 21:556–563.
Article12. Choi AH, Kim J, Chao J. Perioperative chemotherapy for resectable gastric cancer: MAGIC and beyond. World J Gastroenterol. 2015; 21:7343–7348.
Article13. Leong T, Smithers BM, Michael M, Gebski V, Boussioutas A, Miller D, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer. 2015; 15:532.
Article14. Glinski K, Wasilewska-Tesluk E, Rucinska M, Cieslak-Zeranska E, Czeremszynska B, Osowiecka K, et al. Clinical outcome and toxicity of 3D-conformal radiotherapy combined with chemotherapy based on the Intergroup SWOG 9008/INT0116 study protocol for gastric cancer. J BUON. 2015; 20:428–437.15. Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016; 27:suppl 5. v38–v49.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Postoperative Adjuvant Radiotherapy for Patients with Gastric Adenocarcinoma
- Role of Adjuvant Radiotherapy in Gastric Cancer
- Adjuvant Chemotherapy in Gastric Cancer
- Results from the safety interim analysis of the adjuvant chemoradiotherapy in stomach tumors 2 trial: a multicenter, randomized phase III clinical trial
- Update of Adjuvant Chemotherapy for Resected Gastric Cancer