Pediatr Infect Vaccine.
2018 Dec;25(3):132-140. 10.14776/piv.2018.25.e9.
Seroprevalence of Dengue Virus Antibody in Korea
- Affiliations
-
- 1Department of Pediatrics, Ewha Womans University College of Medicine, Seoul, the Republic of Korea. kaykim@ewha.ac.kr
- 2Center for Vaccine Evaluation and Study, Medical Research Institute, Ewha Womans University College of Medicine, Seoul, the Republic of Korea.
- KMID: 2428250
- DOI: http://doi.org/10.14776/piv.2018.25.e9
Abstract
- PURPOSE
The number of dengue fever cases is rising due to increasing overseas travel. Vaccination makes severe dengue fever in seronegative individuals after vaccination when they exposure to wild-type dengue virus. We investigated the seroepidemiology of the dengue virus for monitoring of Korean dengue virus immunity and establishing the prevention of dengue infection.
METHODS
The study was based on 446 residual sera collected from 98 infants (2 months to 1 year old), 152 adolescents (13 to 19 years old), 90 adults (20 to 50 years old), and 106 elderly participants (more than 65 years old) for other studies. Antibody levels for dengue virus immunoglobulin G (IgG) in each age group were measured using an enzyme-linked immunosorbent assay (ELISA). For each dengue virus IgG positive or equivocal result, an IgG ELISA was performed for Japanese encephalitis virus.
RESULTS
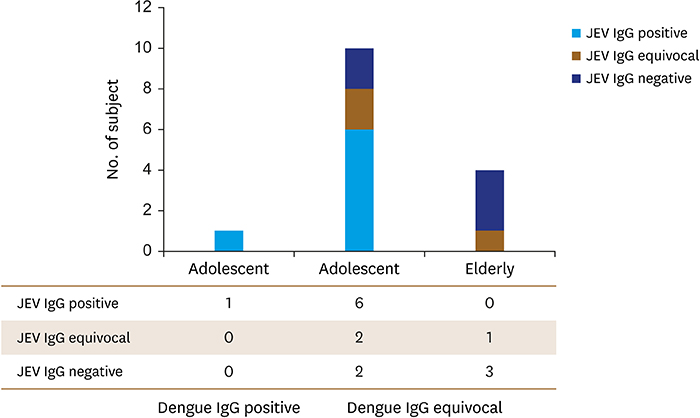
Of the 446 serum samples, only 1 (0.2%) adolescent had a positive result from the dengue IgG antibody test. In the dengue virus IgG antibody test, 14 (3.1%) samples showed equivocal results (10 adolescents and 4 elderly). In the 1 positive case of dengue virus IgG, the Japanese encephalitis IgG test was also positive. In the 14 equivocal cases of dengue virus IgG, there were 6 positive, 3 equivocal, and 5 negative of Japanese encephalitis IgG.
CONCLUSIONS
The seroprevalence rate of dengue virus was very low in Koreans. This study provides important data for establishing the policy for preventive measures of dengue fever. It will be necessary to continuously monitor for dengue virus immunity.
Keyword
MeSH Terms
Figure
Reference
-
1. Nimmannitya S. Clinical spectrum and management of dengue haemorrhagic fever. Southeast Asian J Trop Med Public Health. 1987; 18:392–397.2. L'Azou M, Moureau A, Sarti E, Nealon J, Zambrano B, Wartel TA, et al. Symptomatic dengue in children in 10 Asian and Latin American countries. N Engl J Med. 2016; 374:1155–1166.3. Hales S, de Wet N, Maindonald J, Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet. 2002; 360:830–834.
Article4. Lee SH, Nam KW, Jeong JY, Yoo SJ, Koh YS, Lee S, et al. The effects of climate change and globalization on mosquito vectors: evidence from Jeju Island, South Korea on the potential for Asian tiger mosquito (Aedes albopictus) influxes and survival from Vietnam rather than Japan. PLoS One. 2013; 8:e68512.5. World Health Organization (WHO). Dengue and Severe Dengue [Internet]. Geneva: WHO;2018. cited 2018 May 11. Available from: http://www.who.int/mediacentre/factsheets/fs117/en/.6. Hadinegoro SR, Arredondo-García JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, et al. Efficacy and long-term safety of a Dengue vaccine in regions of endemic disease. N Engl J Med. 2015; 373:1195–1206.
Article7. Flasche S, Jit M, Rodríguez-Barraquer I, Coudeville L, Recker M, Koelle K, et al. The long-term safety, public health impact, and cost-effectiveness of routine vaccination with a recombinant, live-attenuated dengue vaccine (Dengvaxia): a model comparison study. PLoS Med. 2016; 13:e1002181.
Article8. Koh BK, Ng LC, Kita Y, Tang CS, Ang LW, Wong KY, et al. The 2005 dengue epidemic in Singapore: epidemiology, prevention and control. Ann Acad Med Singapore. 2008; 37:538–545.9. Ooi EE, Hart TJ, Tan HC, Chan SH. Dengue seroepidemiology in Singapore. Lancet. 2001; 357:685–686.
Article10. Yew YW, Ye T, Ang LW, Ng LC, Yap G, James L, et al. Seroepidemiology of dengue virus infection among adults in Singapore. Ann Acad Med Singapore. 2009; 38:667–675.11. Raj AS, Munshi S, Shah B. A study on clinical presentation of dengue fever in children. IJSR. 2016; 5:2272–2275.
Article12. Ukey P, Bondade S, Paunipagar P, Powar R, Akulwar S. Study of seroprevalence of dengue fever in central India. Indian J Community Med. 2010; 35:517–519.
Article13. Jamjoom GA, Azhar EI, Kao MA, Radadi RM. Seroepidemiology of asymptomatic Dengue virus infection in Jeddah, Saudi Arabia. Virology (Auckl). 2016; 7:1–7.
Article14. Jeong YE, Kim YH, Cho JE, Han MG, Ju YR. Identification of dengue type 1 virus (DENV-1) in Koreans traveling abroad. Osong Public Health Res Perspect. 2011; 2:34–40.
Article15. Korea Centers for Disease Control and Prevention (KCDCP). Guidelines for Viral Mosquito-born Diseases Prevention and Control [Internet]. Cheongju: KCDCP;2017. cited 2018 May 11. Available from: http://cdc.go.kr/CDC/cms/content/mobile/74/75674_view.html.16. Kim H, Lee C, Kim M. A case of imported dengue hemorrhagic fever. Korean J Infect Dis. 1995; 27:403–406.17. Park JH, Lee DW. Dengue fever in South Korea, 2006–2010. Emerg Infect Dis. 2012; 18:1525–1527.
Article18. Korea Centers for Disease Control and Prevention (KCDCP). Disease Web Statistics System [Internet]. Cheongju: KCDCP;2018. cited 2018 May 11. Available from: https://is.cdc.go.kr/dstat/jsp/stat/stat0001.jsp.19. Korean Tourism Organization. Information on Tourist Statistics [Internet]. Seoul: Korean Tourism Organization;2017. cited 2017 May 11. Available from: http://kto.visitkorea.or.kr/kor/notice/data/statis/profit/board/view.kto?id=379522&isNotice=true&instanceId=294&rnum=0.20. Cobelens FG, Groen J, Osterhaus AD, Leentvaar-Kuipers A, Wertheim-van Dillen PM, Kager PA. Incidence and risk factors of probable dengue virus infection among Dutch travellers to Asia. Trop Med Int Health. 2002; 7:331–338.
Article21. Wilder-Smith A, Schwartz E. Dengue in travelers. N Engl J Med. 2005; 353:924–932.
Article22. Kim DH, Hong YJ, Lee HJ, Choi BY, Kim CH, Park JO, et al. Immunogenicity and protective effectiveness of Japanese Encephalitis vaccine: a prospective multicenter cohort study. Korean J Pediatr Infect Dis. 2013; 20:131–138.
Article23. Lee EJ, Cha GW, Ju YR, Han MG, Lee WJ, Jeong YE. Prevalence of neutralizing antibodies to Japanese Encephalitis virus among high-risk age groups in South Korea, 2010. PLoS One. 2016; 11:e0147841.
Article24. Thai KT, Nga TT, Van Nam N, Phuong HL, Giao PT, Hung Q, et al. Incidence of primary dengue virus infections in Southern Vietnamese children and reactivity against other flaviviruses. Trop Med Int Health. 2007; 12:1553–1557.
Article25. Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989; 40:444–451.
Article26. Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018; 379:327–340.
Article27. Cha GW, Cho JE, Ju YR, Hong YJ, Han MG, Lee WJ, et al. Comparison of four serological tests for detecting antibodies to Japanese encephalitis virus after vaccination in children. Osong Public Health Res Perspect. 2014; 5:286–291.
Article28. Marrero-Santos KM, Beltrán M, Carrión-Lebrón J, Sanchez-Vegas C, Hamer DH, Barnett ED, et al. Optimization of the cutoff value for a commercial anti-dengue virus IgG immunoassay. Clin Vaccine Immunol. 2013; 20:358–362.
Article