Korean J Radiol.
2017 Apr;18(2):323-335. 10.3348/kjr.2017.18.2.323.
Imaging Patterns of Intratumoral Calcification in the Abdominopelvic Cavity
- Affiliations
-
- 1Department of Radiology, Konkuk University Medical Center, Seoul 05030, Korea. yjkim@kuh.ac.kr
- KMID: 2427944
- DOI: http://doi.org/10.3348/kjr.2017.18.2.323
Abstract
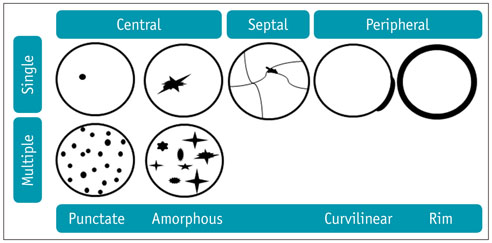
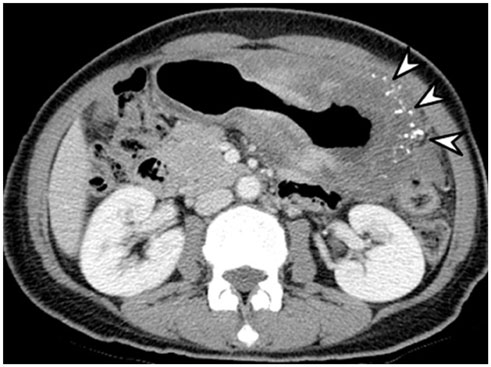
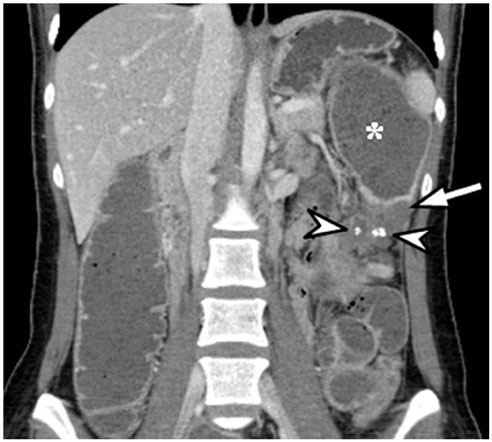
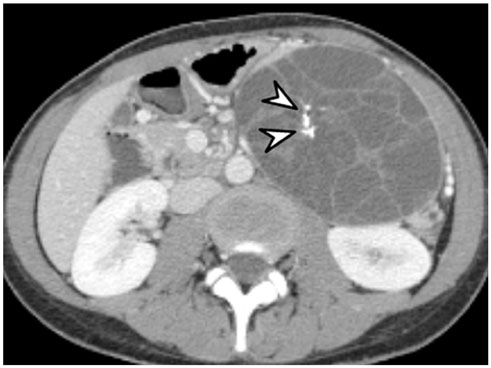
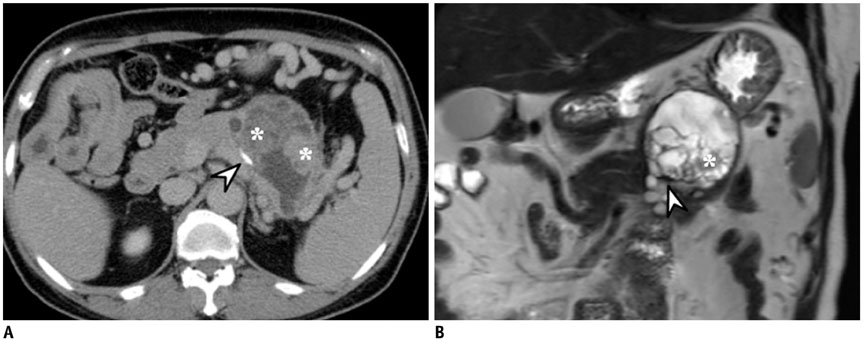
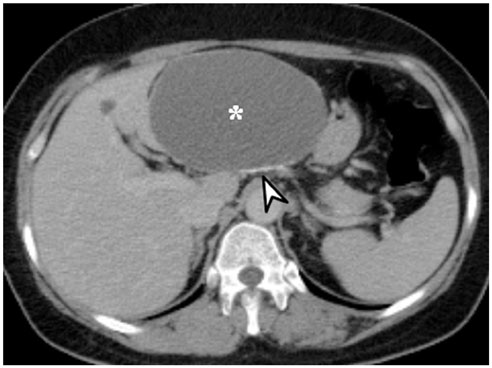
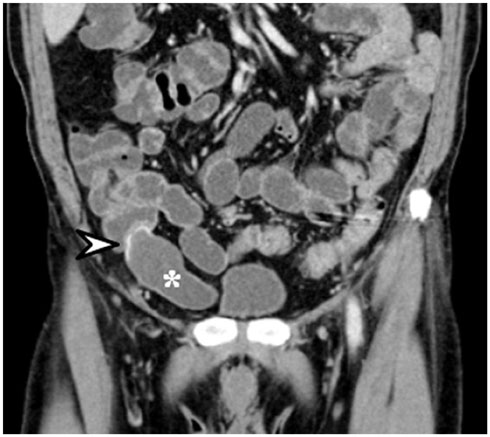
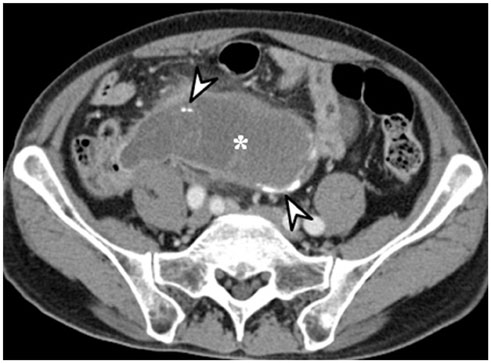
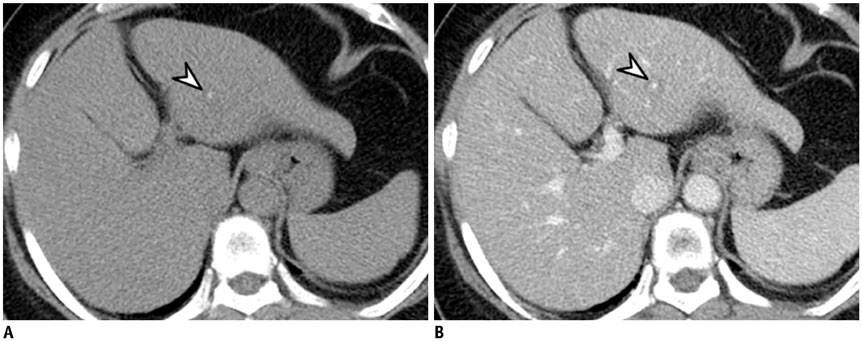
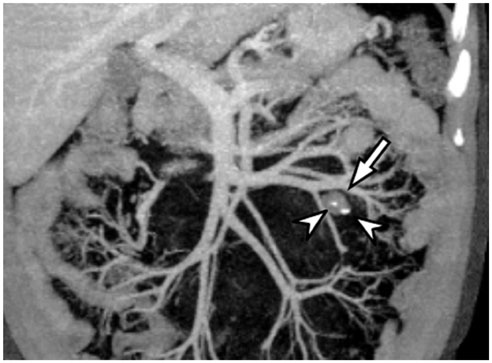
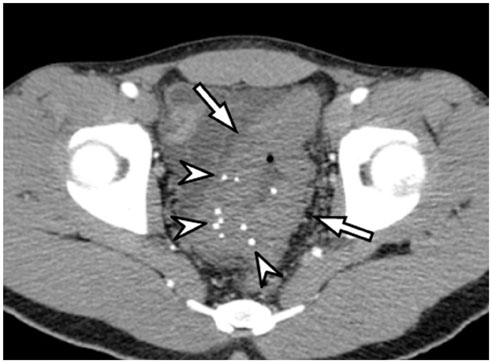
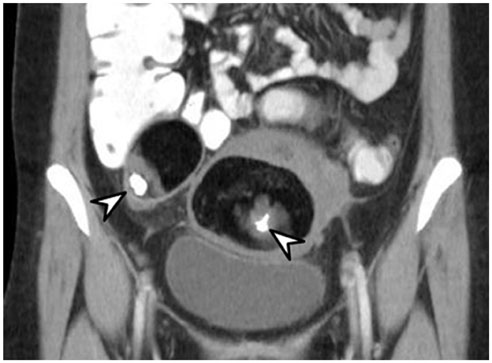
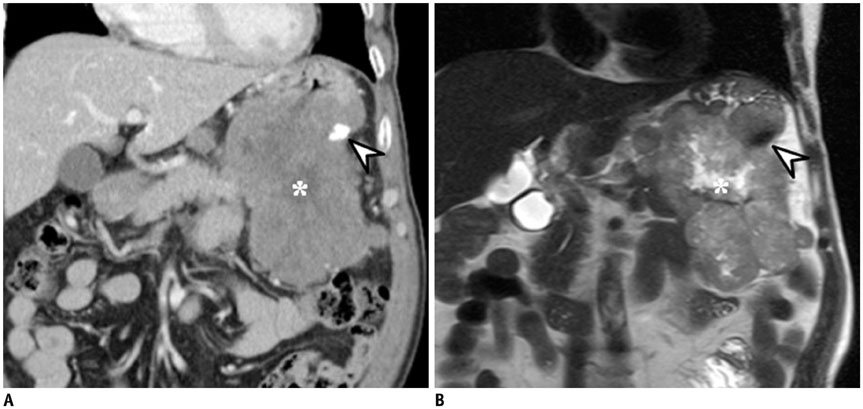
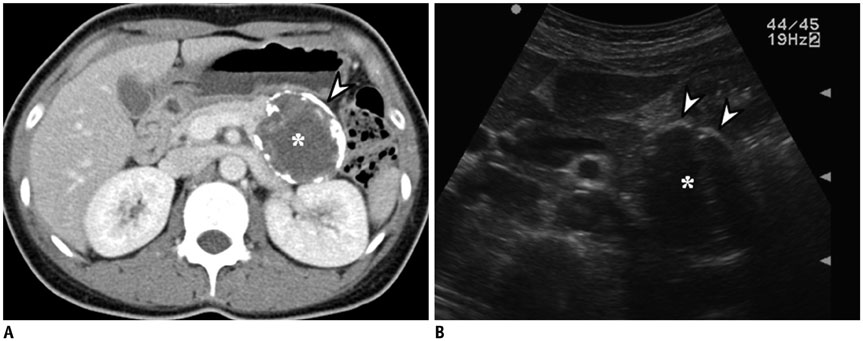
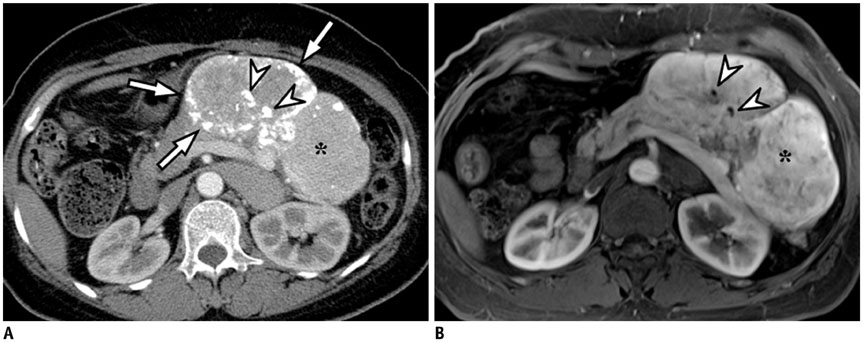
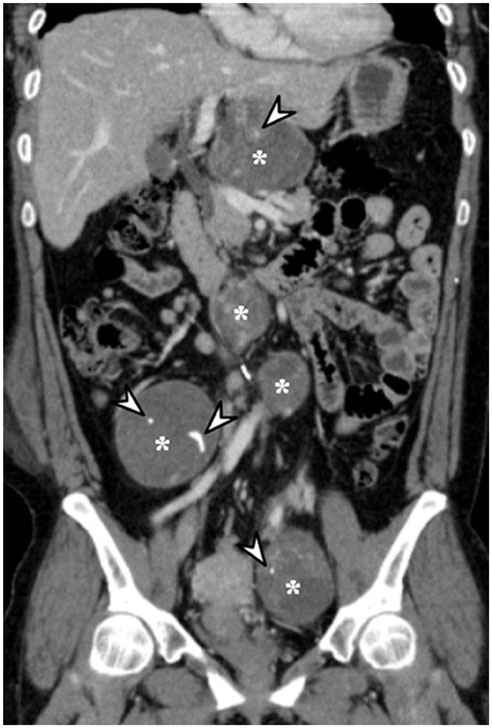
- Intratumoral calcification is one of the most noticeable of radiologic findings. It facilitates detection and provides information important for correctly diagnosing tumors. In the abdominopelvic cavity, a wide variety of tumors have calcifications with various imaging features, though the majority of such calcifications are dystrophic in nature. In this article, we classify the imaging patterns of intratumoral calcification according to number, location, and morphology. Then, we describe commonly-encountered abdominopelvic tumors containing typical calcification patterns, focusing on their differentiable characteristics using the imaging patterns of intratumoral calcification.
Keyword
MeSH Terms
-
Abdominal Neoplasms/complications/*diagnosis/diagnostic imaging
Adenocarcinoma, Mucinous/diagnosis/diagnostic imaging/pathology
Adult
Aged
Calcinosis/complications/*diagnosis/diagnostic imaging
Female
Humans
Image Interpretation, Computer-Assisted
Liver Neoplasms/diagnosis/diagnostic imaging/pathology
Male
Middle Aged
Neoplasm Metastasis
Pancreatic Neoplasms/diagnosis/diagnostic imaging/pathology
Figure
Cited by 1 articles
-
Imaging Evaluation Following 90Y Radioembolization of Liver Tumors: What Radiologists Should Know
Ijin Joo, Hyo-Cheol Kim, Gyoung Min Kim, Jin Chul Paeng
Korean J Radiol. 2018;19(2):209-222. doi: 10.3348/kjr.2018.19.2.209.
Reference
-
1. Curry CA, Eng J, Horton KM, Urban B, Siegelman S, Kuszyk BS, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol. 2000; 175:99–103.2. Ko EY, Ha HK, Kim AY, Yoon KH, Yoo CS, Kim HC, et al. CT differentiation of mucinous and nonmucinous colorectal carcinoma. AJR Am J Roentgenol. 2007; 188:785–791.3. Paley MR, Ros PR. Hepatic calcification. Radiol Clin North Am. 1998; 36:391–398.4. Izawa N, Sawada T, Abiko R, Kumon D, Hirakawa M, Kobayashi M, et al. Gastrointestinal stromal tumor presenting with prominent calcification. World J Gastroenterol. 2012; 18:5645–5648.5. Baek JH, Lee JM, Kim SH, Kim SJ, Kim SH, Lee JY, et al. Small (<or=3 cm) solid pseudopapillary tumors of the pancreas at multiphasic multidetector CT. Radiology. 2010; 257:97–106.6. Giachelli CM. Ectopic calcification: gathering hard facts about soft tissue mineralization. Am J Pathol. 1999; 154:671–675.7. Agarwal A, Yeh BM, Breiman RS, Qayyum A, Coakley FV. Peritoneal calcification: causes and distinguishing features on CT. AJR Am J Roentgenol. 2004; 182:441–445.8. Leyendecker JR, Tchelepi H. Lesion composition. In : Dalrymple NC, Leyendecker JR, Oliphant M, editors. Problem solving in abdominal imaging. 1st ed. Philadephia: Elsevier;2009. p. e1–e20.9. Wu Z, Mittal S, Kish K, Yu Y, Hu J, Haacke EM. Identification of calcification with MRI using susceptibility-weighted imaging: a case study. J Magn Reson Imaging. 2009; 29:177–182.10. Roy B, Verma S, Awasthi R, Rathore RK, Venkatesan R, Yoganathan SA, et al. Correlation of phase values with CT Hounsfield and R2* values in calcified neurocysticercosis. J Magn Reson Imaging. 2011; 34:1060–1064.11. Rahmouni A, Bargoin R, Herment A, Bargoin N, Vasile N. Color Doppler twinkling artifact in hyperechoic regions. Radiology. 1996; 199:269–271.12. Kim HC, Yang DM, Jin W, Ryu JK, Shin HC. Color Doppler twinkling artifacts in various conditions during abdominal and pelvic sonography. J Ultrasound Med. 2010; 29:621–632.13. Sarmiento de la Iglesia MM, Lecumberri Cortés G, Lecumberri Cortés I, Oleaga Zufiria L, Isusi Fontan M, Grande Icaran D. [Intracranial calcifications on MRI]. Radiologia. 2006; 48:19–26.14. Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012; 3:251–261.15. Park MS, Yu JS, Kim MJ, Yoon SW, Kim SH, Noh TW, et al. Mucinous versus nonmucinous gastric carcinoma: differentiation with helical CT. Radiology. 2002; 223:540–546.16. Ghahremani GG, Meyers MA, Port RB. Calcified primary tumors of the gastrointestinal tract. Gastrointest Radiol. 1978; 2:331–339.17. Stoupis C, Taylor HM, Paley MR, Buetow PC, Marre S, Baer HU, et al. The Rocky liver: radiologic-pathologic correlation of calcified hepatic masses. Radiographics. 1998; 18:675–685. quiz 726.18. Bosman FT, Cameron JL, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press;2010. p. 217–224. p. 236–238. p. 254–261.19. Kim HJ, Yu ES, Byun JH, Hong SM, Kim KW, Lee JS, et al. CT differentiation of mucin-producing cystic neoplasms of the liver from solitary bile duct cysts. AJR Am J Roentgenol. 2014; 202:83–91.20. Farrell JJ. Prevalence, diagnosis and management of pancreatic cystic neoplasms: current status and future directions. Gut Liver. 2015; 9:571–589.21. Buetow PC, Rao P, Thompson LD. From the Archives of the AFIP. Mucinous cystic neoplasms of the pancreas: radiologic-pathologic correlation. Radiographics. 1998; 18:433–449.22. Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005; 25:1471–1484.23. Lee NK, Kim S, Kim HS, Jeon TY, Kim GH, Kim DU, et al. Spectrum of mucin-producing neoplastic conditions of the abdomen and pelvis: cross-sectional imaging evaluation. World J Gastroenterol. 2011; 17:4757–4771.24. Qian LJ, Zhu J, Zhuang ZG, Xia Q, Liu Q, Xu JR. Spectrum of multilocular cystic hepatic lesions: CT and MR imaging findings with pathologic correlation. Radiographics. 2013; 33:1419–1433.25. Buetow PC, Buck JL, Pantongrag-Brown L, Ros PR, Devaney K, Goodman ZD, et al. Biliary cystadenoma and cystadenocarcinoma: clinical-imaging-pathologic correlations with emphasis on the importance of ovarian stroma. Radiology. 1995; 196:805–810.26. Pickhardt PJ, Levy AD, Rohrmann CA Jr, Kende AI. Primary neoplasms of the appendix: radiologic spectrum of disease with pathologic correlation. Radiographics. 2003; 23:645–662.27. Carr N, Sobin L. Tumors of the appendix. In : Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press;2010. p. 122–125.28. Tirumani SH, Fraser-Hill M, Auer R, Shabana W, Walsh C, Lee F, et al. Mucinous neoplasms of the appendix: a current comprehensive clinicopathologic and imaging review. Cancer Imaging. 2013; 13:14–25.29. Madwed D, Mindelzun R, Jeffrey RB Jr. Mucocele of the appendix: imaging findings. AJR Am J Roentgenol. 1992; 159:69–72.30. Persaud T, Swan N, Torreggiani WC. Giant mucinous cystadenoma of the appendix. Radiographics. 2007; 27:553–557.31. Hale HL, Husband JE, Gossios K, Norman AR, Cunningham D. CT of calcified liver metastases in colorectal carcinoma. Clin Radiol. 1998; 53:735–741.32. Easson AM, Barron PT, Cripps C, Hill G, Guindi M, Michaud C. Calcification in colorectal hepatic metastases correlates with longer survival. J Surg Oncol. 1996; 63:221–225.33. Caseiro-Alves F, Brito J, Araujo AE, Belo-Soares P, Rodrigues H, Cipriano A, et al. Liver haemangioma: common and uncommon findings and how to improve the differential diagnosis. Eur Radiol. 2007; 17:1544–1554.34. Vilgrain V, Boulos L, Vullierme MP, Denys A, Terris B, Menu Y. Imaging of atypical hemangiomas of the liver with pathologic correlation. Radiographics. 2000; 20:379–397.35. Klotz T, Montoriol PF, Da Ines D, Petitcolin V, Joubert-Zakeyh J, Garcier JM. Hepatic haemangioma: common and uncommon imaging features. Diagn Interv Imaging. 2013; 94:849–859.36. Mitsudo K, Watanabe Y, Saga T, Dohke M, Sato N, Minami K, et al. Nonenhanced hepatic cavernous hemangioma with multiple calcifications: CT and pathologic correlation. Abdom Imaging. 1995; 20:459–461.37. Djouhri H, Arrivé L, Bouras T, Martin B, Monnier-Cholley L, Tubiana JM. Diffuse cavernous hemangioma of the rectosigmoid colon: imaging findings. J Comput Assist Tomogr. 1998; 22:851–855.38. Yoo S. GI-associated hemangiomas and vascular malformations. Clin Colon Rectal Surg. 2011; 24:193–200.39. Levy AD, Abbott RM, Rohrmann CA Jr, Frazier AA, Kende A. Gastrointestinal hemangiomas: imaging findings with pathologic correlation in pediatric and adult patients. AJR Am J Roentgenol. 2001; 177:1073–1081.40. Hsu RM, Horton KM, Fishman EK. Diffuse cavernous hemangiomatosis of the colon: findings on three-dimensional CT colonography. AJR Am J Roentgenol. 2002; 179:1042–1044.41. Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001; 21:475–490.42. Park SB, Kim JK, Kim KR, Cho KS. Imaging findings of complications and unusual manifestations of ovarian teratomas. Radiographics. 2008; 28:969–983.43. Jung SE, Lee JM, Rha SE, Byun JY, Jung JI, Hahn ST. CT and MR imaging of ovarian tumors with emphasis on differential diagnosis. Radiographics. 2002; 22:1305–1325.44. Guinet C, Ghossain MA, Buy JN, Malbec L, Hugol D, Truc JB, et al. Mature cystic teratomas of the ovary: CT and MR findings. Eur J Radiol. 1995; 20:137–143.45. Buy JN, Ghossain MA, Moss AA, Bazot M, Doucet M, Hugol D, et al. Cystic teratoma of the ovary: CT detection. Radiology. 1989; 171:697–701.46. Friedman AC, Pyatt RS, Hartman DS, Downey EF Jr, Olson WB. CT of benign cystic teratomas. AJR Am J Roentgenol. 1982; 138:659–665.47. Saba L, Guerriero S, Sulcis R, Virgilio B, Melis G, Mallarini G. Mature and immature ovarian teratomas: CT, US and MR imaging characteristics. Eur J Radiol. 2009; 72:454–463.48. Moon W, Kim Y, Rhim H, Koh B, Cho O. Coexistent cystic teratoma of the omentum and ovary: report of two cases. Abdom Imaging. 1997; 22:516–518.49. Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003; 23:283–304. 456quiz 532.50. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006; 23:70–83.51. Ghanem N, Altehoefer C, Furtwängler A, Winterer J, Schäfer O, Springer O, et al. Computed tomography in gastrointestinal stromal tumors. Eur Radiol. 2003; 13:1669–1678.52. Tateishi U, Hasegawa T, Satake M, Moriyama N. Gastrointestinal stromal tumor. Correlation of computed tomography findings with tumor grade and mortality. J Comput Assist Tomogr. 2003; 27:792–798.53. Kim HC, Lee JM, Choi SH, Kim KW, Kim SH, Lee JY, et al. Imaging of gastrointestinal stromal tumors. J Comput Assist Tomogr. 2004; 28:596–604.54. Law JK, Ahmed A, Singh VK, Akshintala VS, Olson MT, Raman SP, et al. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas. 2014; 43:331–337.55. Choi JY, Kim MJ, Kim JH, Kim SH, Lim JS, Oh YT, et al. Solid pseudopapillary tumor of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol. 2006; 187:W178–W186.56. Buetow PC, Buck JL, Pantongrag-Brown L, Beck KG, Ros PR, Adair CF. Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation on 56 cases. Radiology. 1996; 199:707–711.57. Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut. 2012; 61:6–32.58. Sahani DV, Bonaffini PA, Fernández-Del Castillo C, Blake MA. Gastroenteropancreatic neuroendocrine tumors: role of imaging in diagnosis and management. Radiology. 2013; 266:38–61.59. Heller MT, Shah AB. Imaging of neuroendocrine tumors. Radiol Clin North Am. 2011; 49:529–548. vii60. Lewis RB, Lattin GE Jr, Paal E. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics. 2010; 30:1445–1464.61. Buetow PC, Parrino TV, Buck JL, Pantongrag-Brown L, Ros PR, Dachman AH, et al. Islet cell tumors of the pancreas: pathologic-imaging correlation among size, necrosis and cysts, calcification, malignant behavior, and functional status. AJR Am J Roentgenol. 1995; 165:1175–1179.62. Poultsides GA, Huang LC, Chen Y, Visser BC, Pai RK, Jeffrey RB, et al. Pancreatic neuroendocrine tumors: radiographic calcifications correlate with grade and metastasis. Ann Surg Oncol. 2012; 19:2295–2303.63. Kim DW, Kim HJ, Kim KW, Byun JH, Song KB, Kim JH, et al. Neuroendocrine neoplasms of the pancreas at dynamic enhanced CT: comparison between grade 3 neuroendocrine carcinoma and grade 1/2 neuroendocrine tumour. Eur Radiol. 2015; 25:1375–1383.64. Low G, Panu A, Millo N, Leen E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics. 2011; 31:993–1015.65. Rha SE, Byun JY, Jung SE, Chun HJ, Lee HG, Lee JM. Neurogenic tumors in the abdomen: tumor types and imaging characteristics. Radiographics. 2003; 23:29–43.66. Kim TJ, Han JK, Kim YH, Kim TK, Choi BI. Castleman disease of the abdomen: imaging spectrum and clinicopathologic correlations. J Comput Assist Tomogr. 2001; 25:207–214.67. Ko SF, Hsieh MJ, Ng SH, Lin JW, Wan YL, Lee TY, et al. Imaging spectrum of Castleman's disease. AJR Am J Roentgenol. 2004; 182:769–775.68. Bonekamp D, Horton KM, Hruban RH, Fishman EK. Castleman disease: the great mimic. Radiographics. 2011; 31:1793–1807.69. Meador TL, McLarney JK. CT features of Castleman disease of the abdomen and pelvis. AJR Am J Roentgenol. 2000; 175:115–118.70. Hill AJ, Tirumani SH, Rosenthal MH, Shinagare AB, Carrasco RD, Munshi NC, et al. Multimodality imaging and clinical features in Castleman disease: single institute experience in 30 patients. Br J Radiol. 2015; 88:20140670.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CT Analysis of Intratumoral Gas Formation after Hepatic Tumor Embolization
- Imaging Findings of Pediatric Oligodendroglioma
- Multifocal Peritoneal Splenosis in Tc-99m-Labeled Heat-Denatured Red Blood Cell Scintigraphy
- Radiological observation of determination of sex by costal cartilage calcification
- Chondrosarcoma with Intratumoral Hemorrhage: Case Report