Korean J Radiol.
2017 Apr;18(2):289-298. 10.3348/kjr.2017.18.2.289.
The Effects of Breathing Motion on DCE-MRI Images: Phantom Studies Simulating Respiratory Motion to Compare CAIPIRINHA-VIBE, Radial-VIBE, and Conventional VIBE
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea. kyungwon_kim@amc.seoul.kr
- 2Department of Radiology, Yonsei University College of Medicine, Severance Hospital, Seoul 03722, Korea.
- 3Department of Radiology, Ajou Unversity School of Medicine, Suwon 16499, Korea.
- 4Department of Radiology, Ulsan University Hospital, Ulsan 44033, Korea.
- 5Siemens Healthcare Korea, Seoul 03737, Korea.
- 6MR Application Predevelopment, Siemens Healthcare, Erlangen 91052, Germany.
- KMID: 2427941
- DOI: http://doi.org/10.3348/kjr.2017.18.2.289
Abstract
OBJECTIVE
To compare the breathing effects on dynamic contrast-enhanced (DCE)-MRI between controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA)-volumetric interpolated breath-hold examination (VIBE), radial VIBE with k-space-weighted image contrast view-sharing (radial-VIBE), and conventional VIBE (c-VIBE) sequences using a dedicated phantom experiment.
MATERIALS AND METHODS
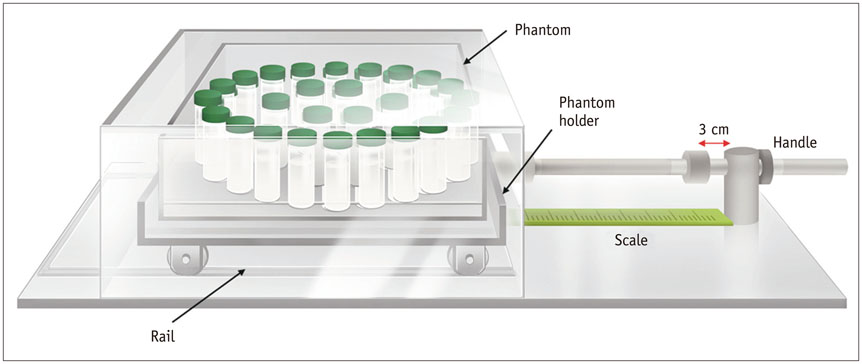
We developed a moving platform to simulate breathing motion. We conducted dynamic scanning on a 3T machine (MAGNETOM Skyra, Siemens Healthcare) using CAIPIRINHA-VIBE, radial-VIBE, and c-VIBE for six minutes per sequence. We acquired MRI images of the phantom in both static and moving modes, and we also obtained motion-corrected images for the motion mode. We compared the signal stability and signal-to-noise ratio (SNR) of each sequence according to motion state and used the coefficients of variation (CoV) to determine the degree of signal stability.
RESULTS
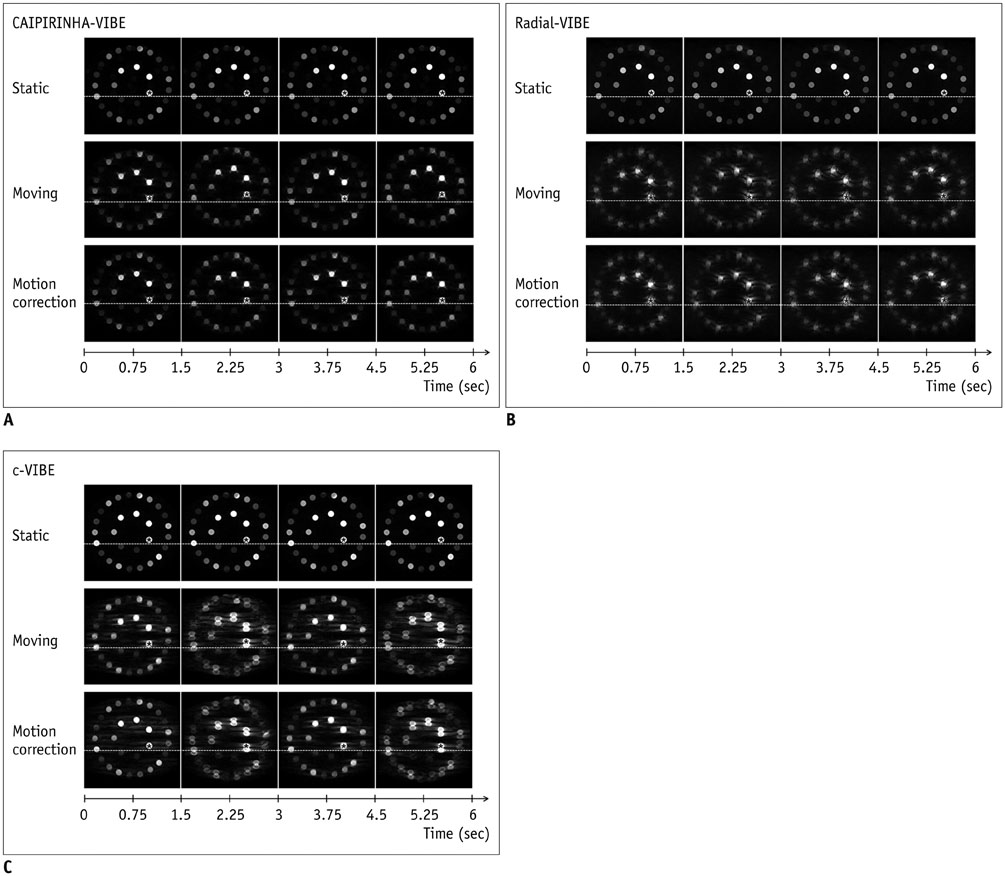
With motion, CAIPIRINHA-VIBE showed the best image quality, and the motion correction aligned the images very well. The CoV (%) of CAIPIRINHA-VIBE in the moving mode (18.65) decreased significantly after the motion correction (2.56) (p < 0.001). In contrast, c-VIBE showed severe breathing motion artifacts that did not improve after motion correction. For radial-VIBE, the position of the phantom in the images did not change during motion, but streak artifacts significantly degraded image quality, also after motion correction. In addition, SNR increased in both CAIPIRINHA-VIBE (from 3.37 to 9.41, p < 0.001) and radial-VIBE (from 4.3 to 4.96, p < 0.001) after motion correction.
CONCLUSION
CAIPIRINHA-VIBE performed best for free-breathing DCE-MRI after motion correction, with excellent image quality.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Rapid Imaging: Recent Advances in Abdominal MRI for Reducing Acquisition Time and Its Clinical Applications
Jeong Hee Yoon, Marcel Dominik Nickel, Johannes M. Peeters, Jeong Min Lee
Korean J Radiol. 2019;20(12):1597-1615. doi: 10.3348/kjr.2018.0931.
Reference
-
1. Thng CH, Koh TS, Collins DJ, Koh DM. Perfusion magnetic resonance imaging of the liver. World J Gastroenterol. 2010; 16:1598–1609.2. Kim KW, Lee JM, Jeon YS, Kang SE, Baek JH, Han JK, et al. Free-breathing dynamic contrast-enhanced MRI of the abdomen and chest using a radial gradient echo sequence with k-space weighted image contrast (KWIC). Eur Radiol. 2013; 23:1352–1360.3. Kim B, Lee CK, Seo N, Lee SS, Kim JK, Choi Y, et al. Comparison of CAIPIRINHA-VIBE, Radial-VIBE, and conventional VIBE sequences for dynamic contrast-enhanced (DCE) MRI: a validation study using a DCE-MRI phantom. Magn Reson Imaging. 2016; 34:638–644.4. Hintze C, Stemmer A, Bock M, Kuder TA, Risse F, Dinkel J, et al. A hybrid breath hold and continued respiration-triggered technique for time-resolved 3D MRI perfusion studies in lung cancer. Rofo. 2010; 182:45–52.5. Breuer FA, Blaimer M, Mueller MF, Seiberlich N, Heidemann RM, Griswold MA, et al. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn Reson Med. 2006; 55:549–556.6. McKenzie CA, Lim D, Ransil BJ, Morrin M, Pedrosa I, Yeh EN, et al. Shortening MR image acquisition time for volumetric interpolated breath-hold examination with a recently developed parallel imaging reconstruction technique: clinical feasibility. Radiology. 2004; 230:589–594.7. Vogt FM, Antoch G, Hunold P, Maderwald S, Ladd ME, Debatin JF, et al. Parallel acquisition techniques for accelerated volumetric interpolated breath-hold examination magnetic resonance imaging of the upper abdomen: assessment of image quality and lesion conspicuity. J Magn Reson Imaging. 2005; 21:376–382.8. AlObaidy M, Ramalho M, Busireddy KK, Liu B, Burke LM, Altun E, et al. High-resolution 3D-GRE imaging of the abdomen using controlled aliasing acceleration technique-a feasibility study. Eur Radiol. 2015; 25:3596–3605.9. Jackson E. Technical Committee MR Phantom Development/Data Efforts. Web site. Accessed January 21, 2017. http://qibawiki.rsna.org/images/3/34/RSNA_QIBA_May2010_MR_Phantom.pdf.10. Lin W, Guo J, Rosen MA, Song HK. Respiratory motion-compensated radial dynamic contrast-enhanced (DCE)-MRI of chest and abdominal lesions. Magn Reson Med. 2008; 60:1135–1146.11. Chandarana H, Block TK, Rosenkrantz AB, Lim RP, Kim D, Mossa DJ, et al. Free-breathing radial 3D fat-suppressed T1-weighted gradient echo sequence: a viable alternative for contrast-enhanced liver imaging in patients unable to suspend respiration. Invest Radiol. 2011; 46:648–653.12. Song HK, Dougherty L. Dynamic MRI with projection reconstruction and KWIC processing for simultaneous high spatial and temporal resolution. Magn Reson Med. 2004; 52:815–824.13. Song HK, Dougherty L. k-space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magn Reson Med. 2000; 44:825–832.14. Chefd'hotel C, Hermosillo G, Faugeras O. Flows of diffeomorphisms for multimodal image registration. In : Proceedings of the IEEE International Symposium on Biomedical Imaging; Piscataway, NJ: Institute of Electrical and Electronics Engineers;2002. p. 753–756.15. Reed GF, Lynn F, Meade BD. Use of coefficient of variation in assessing variability of quantitative assays. Clin Diagn Lab Immunol. 2002; 9:1235–1239.16. Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging. 2007; 26:375–385.17. Sodickson DK, Griswold MA, Jakob PM, Edelman RR, Manning WJ. Signal-to-noise ratio and signal-to-noise efficiency in SMASH imaging. Magn Reson Med. 1999; 41:1009–1022.18. Reeder SB, Wintersperger BJ, Dietrich O, Lanz T, Greiser A, Reiser MF, et al. Practical approaches to the evaluation of signal-to-noise ratio performance with parallel imaging: application with cardiac imaging and a 32-channel cardiac coil. Magn Reson Med. 2005; 54:748–754.19. Yu MH, Lee JM, Yoon JH, Kiefer B, Han JK, Choi BI. Clinical application of controlled aliasing in parallel imaging results in a higher acceleration (CAIPIRINHA)-volumetric interpolated breathhold (VIBE) sequence for gadoxetic acid-enhanced liver MR imaging. J Magn Reson Imaging. 2013; 38:1020–1026.20. Block KT, Uecker M, Frahm J. Undersampled radial MRI with multiple coils. Iterative image reconstruction using a total variation constraint. Magn Reson Med. 2007; 57:1086–1098.21. Fujinaga Y, Ohya A, Tokoro H, Yamada A, Ueda K, Ueda H, et al. Radial volumetric imaging breath-hold examination (VIBE) with k-space weighted image contrast (KWIC) for dynamic gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI of the liver: advantages over Cartesian VIBE in the arterial phase. Eur Radiol. 2014; 24:1290–1299.22. Haider CR, Riederer SJ, Borisch EA, Glockner JF, Grimm RC, Hulshizer TC, et al. High temporal and spatial resolution 3D time-resolved contrast-enhanced magnetic resonance angiography of the hands and feet. J Magn Reson Imaging. 2011; 34:2–12.23. Kim BS, Lee KR, Goh MJ. New imaging strategies using a motion-resistant liver sequence in uncooperative patients. Biomed Res Int. 2014; 2014:142658.24. Clifford MA, Banovac F, Levy E, Cleary K. Assessment of hepatic motion secondary to respiration for computer assisted interventions. Comput Aided Surg. 2002; 7:291–299.25. Suramo I, Päivänsalo M, Myllylä V. Cranio-caudal movements of the liver, pancreas and kidneys in respiration. Acta Radiol Diagn (Stockh). 1984; 25:129–131.26. Korin HW, Ehman RL, Riederer SJ, Felmlee JP, Grimm RC. Respiratory kinematics of the upper abdominal organs: a quantitative study. Magn Reson Med. 1992; 23:172–178.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphologic Evaluation of Primary Non-Small Cell Lung Cancer by 3 Tesla MRI with Free-Breathing Ultrashort Echo Time and Radial T1-Weighted Gradient Echo Sequences: A Comparison with CT Analysis
- Contrast-Enhanced Three-Dimensional MR Imaging Using a Volumetric Interpolated Breath-hold Examination (VIBE): Clinical Utility in the Evaluation of Renal Tumors
- Feasibility of Quadruple Arterial Phase of Motion Insensitive Radial Volumetric Imaging Breath-Hold Examination with k-Space Weighted Image Contrast in the Detection of Hepatocellular Carcinoma in Patients with Chronic Liver Disease
- The Usefulness of High-Resolution Three-Dimensional Dynamic MR Imaging with Sensitivity Encoding for Evaluating Extrahepatic Bile Duct Cancer
- Bendamustine in combination with ifosfamide, etoposide, and vinorelbine (VIBE) is an effective salvage regimen for heavily pre-treated patients with relapsed or refractory Hodgkin lymphoma: a single-center experience