Ann Hepatobiliary Pancreat Surg.
2018 Nov;22(4):405-411. 10.14701/ahbps.2018.22.4.405.
The survival impact of surgical waiting time in patients with resectable pancreatic head cancer
- Affiliations
-
- 1Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. dwhwang@amc.seoul.kr
- 2Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2427373
- DOI: http://doi.org/10.14701/ahbps.2018.22.4.405
Abstract
- BACKGROUNDS/AIMS
After centralization policy, clinical outcomes have been improved in patients underwent pancreaticoduodenectomy for pancreatic cancer. However, centralization could exacerbate the prolongation of surgical waiting time. This study aims to investigate whether the shorter waiting time correlates with the better survival and to identify the major confounders that influence the association between those.
METHODS
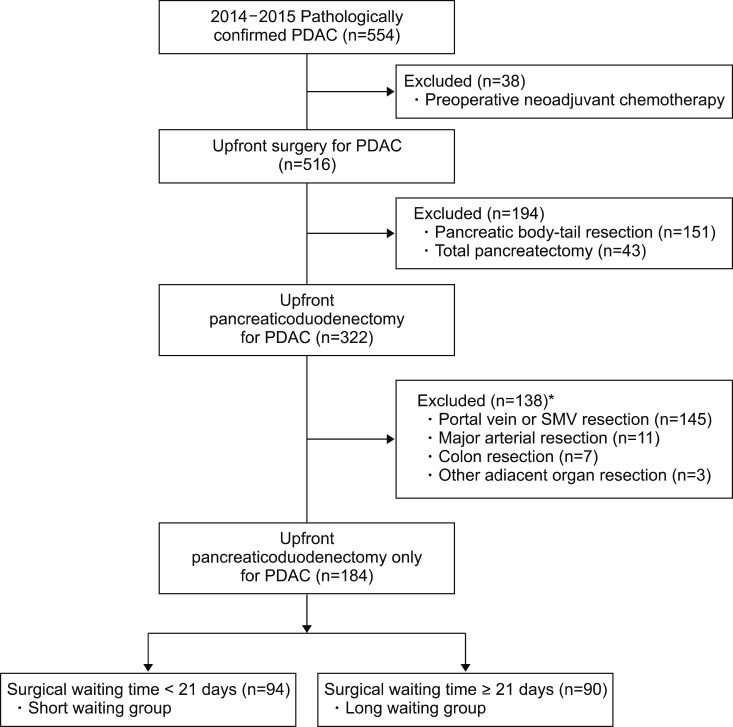
In this retrospective cohort study, a total 554 patients with pathologically confirmed pancreatic ductal adenocarcinoma were assessed the eligibility from 2014 through 2015. Patients with neoadjuvant chemotherapy, body-tail resection, total pancreatectomy and combined adjacent organ resection were excluded. All patients were divided into two groups by median waiting time, 21 days, defined as the date difference between initial imaging diagnosis and operation.
RESULTS
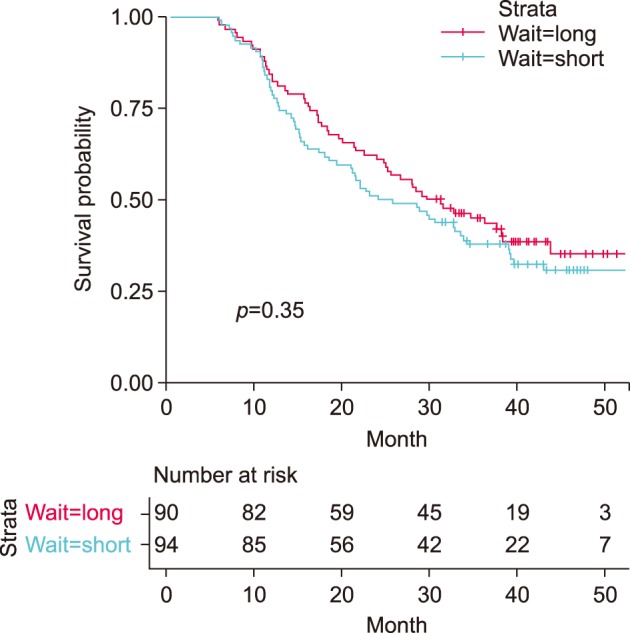
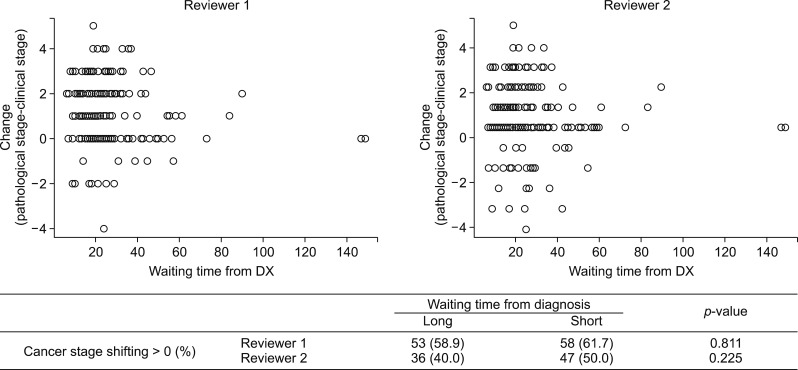
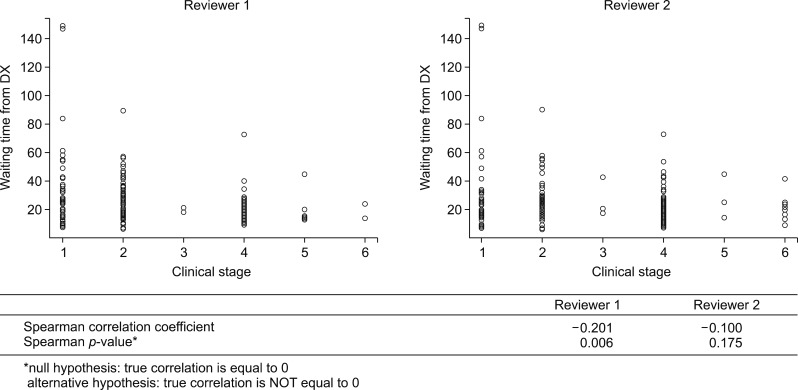
Median overall survival did not differ between long and short waiting group (30.4 vs 24.8 months, p=0.35; HR=0.84, 95% CI=0.58-1.21). The proportion of cancer stage shifting, the difference between clinical and pathologic staging, did not differ depending on waiting time group (p=0.811 and 0.255, each of reviewers). Short waiting time was highly correlated with high initial clinical stage (Spearman correlation coefficients −0.201 (p=0.006) and −0.100 (p=0.175), each of reviewers).
CONCLUSIONS
Initial clinical stage had confounding effect on the association between waiting time and overall survival. Therefore, in evaluating centralization policy at the national level, evidence for maximum acceptable waiting time should be investigated in the near future with considering that surgical waiting time could be affected by initial clinical stage.
MeSH Terms
Figure
Reference
-
1. Shin SH, Kim SC, Song KB, Hwang DW, Lee JH, Park KM, et al. Chronologic changes in clinical and survival features of pancreatic ductal adenocarcinoma since 2000: a single-center experience with 2,029 patients. Surgery. 2018; 164:432–442. PMID: 29884479.
Article2. Hartwig W, Werner J, Jäger D, Debus J, Büchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013; 14:e476–e485. PMID: 24079875.
Article3. van Heek NT, Kuhlmann KF, Scholten RJ, de Castro SM, Busch OR, van Gulik TM, et al. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005; 242:781–788. PMID: 16327488.4. Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011; 49:1076–1081. PMID: 22002649.
Article5. Christensen ED, Harvald T, Jendresen M, Aggestrup S, Petterson G. The impact of delayed diagnosis of lung cancer on the stage at the time of operation. Eur J Cardiothorac Surg. 1997; 12:880–884. PMID: 9489874.
Article6. Langenbach MR, Schmidt J, Neumann J, Zirngibl H. Delay in treatment of colorectal cancer: multifactorial problem. World J Surg. 2003; 27:304–308. PMID: 12607056.
Article7. Sainsbury R, Johnston C, Haward B. Effect on survival of delays in referral of patients with breast-cancer symptoms: a retrospective analysis. Lancet. 1999; 353:1132–1135. PMID: 10209976.
Article8. Marchegiani G, Andrianello S, Perri G, Secchettin E, Maggino L, Malleo G, et al. Does the surgical waiting list affect pathological and survival outcome in resectable pancreatic ductal adenocarcinoma? HPB (Oxford). 2018; 20:411–417. PMID: 29191689.
Article9. Sanjeevi S, Ivanics T, Lundell L, Kartalis N, Andrén-Sandberg Å, Blomberg J, et al. Impact of delay between imaging and treatment in patients with potentially curable pancreatic cancer. Br J Surg. 2016; 103:267–275. PMID: 26572509.
Article10. Yun YH, Kim YA, Min YH, Park S, Won YJ, Kim DY, et al. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann Oncol. 2012; 23:2731–2737. PMID: 22553194.
Article11. Hong DP, Song J. The effective distribution system for the concentration of patients to extra-large hospitals. J Korean Surg Soc. 2011; 80:373–383. PMID: 22066063.
Article12. Marchegiani G, Andrianello S, Malleo G, De Gregorio L, Scarpa A, Mino-Kenudson M, et al. Does size matter in pancreatic cancer?: reappraisal of tumour dimension as a predictor of outcome beyond the TNM. Ann Surg. 2017; 266:142–148. PMID: 27322188.
Article13. Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008; 9:99–132. PMID: 18326920.14. McLean SR, Karsanji D, Wilson J, Dixon E, Sutherland FR, Pasieka J, et al. The effect of wait times on oncological outcomes from periampullary adenocarcinomas. J Surg Oncol. 2013; 107:853–858. PMID: 23625192.
Article15. Healy GM, Redmond CE, Murphy S, Fleming H, Haughey A, Kavanagh R, et al. Preoperative CT in patients with surgically resectable pancreatic adenocarcinoma: does the time interval between CT and surgery affect survival? Abdom Radiol (NY). 2018; 43:620–628. PMID: 28695235.
Article16. Pieper D, Mathes T, Neugebauer E, Eikermann M. State of evidence on the relationship between high-volume hospitals and outcomes in surgery: a systematic review of systematic reviews. J Am Coll Surg. 2013; 216:1015–1025.e18. PMID: 23528183.
Article17. Hata T, Motoi F, Ishida M, Naitoh T, Katayose Y, Egawa S, et al. Effect of hospital volume on surgical outcomes after pancreaticoduodenectomy: a systematic review and meta-analysis. Ann Surg. 2016; 263:664–672. PMID: 26636243.18. Balzano G, Zerbi A, Capretti G, Rocchetti S, Capitanio V, Di Carlo V. Effect of hospital volume on outcome of pancreaticoduodenectomy in Italy. Br J Surg. 2008; 95:357–362. PMID: 17933001.
Article19. BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax. 1998; 53(Suppl 1):S1–S8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant Therapy for Resectable or Borderline Resectable Pancreatic Cancer
- Updates of Chemotherapy for Pancreatic Cancer
- Updates of Chemotherapy and Radiotherapy for Pancreatic Cancer
- Pancreas club international joint symposium on pancreatic cancer 2012, Kyoto: down staging chemo+/-radiotherapy for borderline resectable pancreatic cancer
- Longer waiting times for early stage cervical cancer patients undergoing radical hysterectomy are associated with diminished long-term overall survival