Yonsei Med J.
2016 Nov;57(6):1494-1499. 10.3349/ymj.2016.57.6.1494.
Troglitazone Enhances the Apoptotic Response of DLD-1 Colon Cancer Cells to Photodynamic Therapy
- Affiliations
-
- 1Department of Physiology, Tissue Injury Defense Research Center, Ewha Womans University School of Medicine, Seoul, Korea. yc@ewha.ac.kr
- 2Department of Microbiology, Institute for Immunology and Immunological Diseases, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2427170
- DOI: http://doi.org/10.3349/ymj.2016.57.6.1494
Abstract
- PURPOSE
The aim of this study was to investigate whether the peroxisomal proliferator-activated receptor gamma (PPARγ) ligand troglitazone in combination with photodynamic therapy (PDT) enhances the apoptotic response of DLD-1 colon cancer cells.
MATERIALS AND METHODS
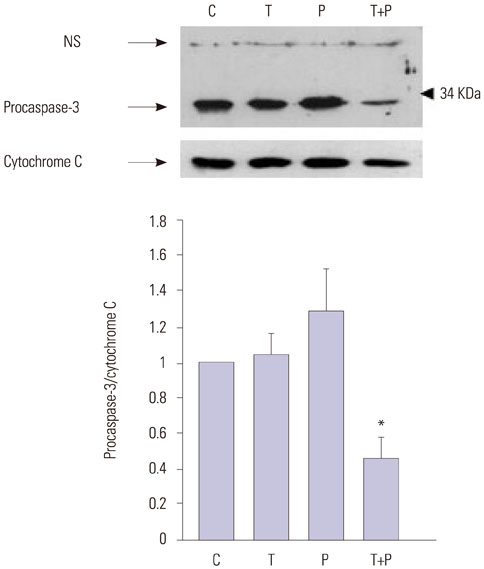
The effects of troglitazone, PDT, and troglitazone in combination with PDT on cell viability and apoptosis were assessed in DLD-1 cells. Cell viability and proliferation were evaluated using the tetrazolium-based MTT assay, and apoptosis was evaluated via cell staining with propidium iodide (PI) and annexin V-FITC. The levels of pro-caspase-3 were measured via Western blot analyses.
RESULTS
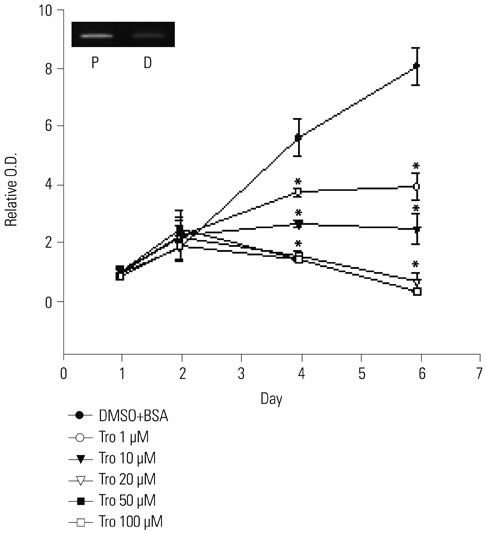
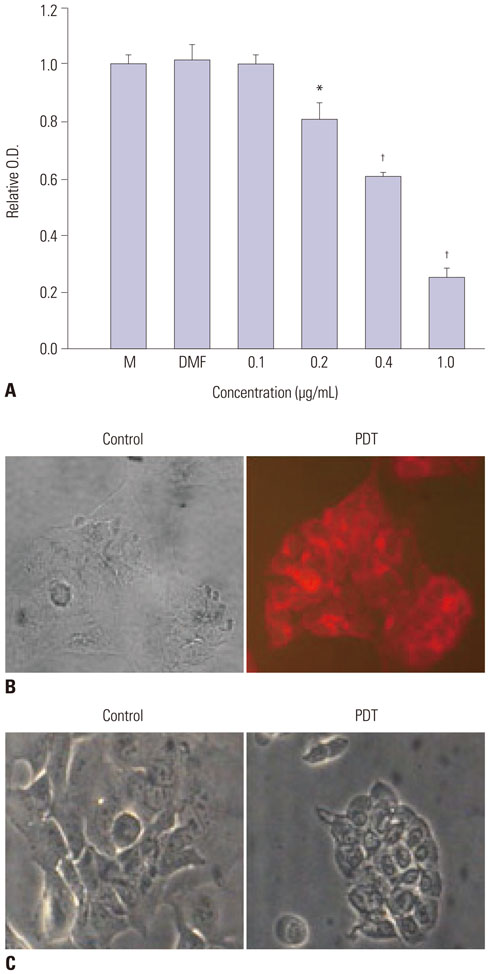
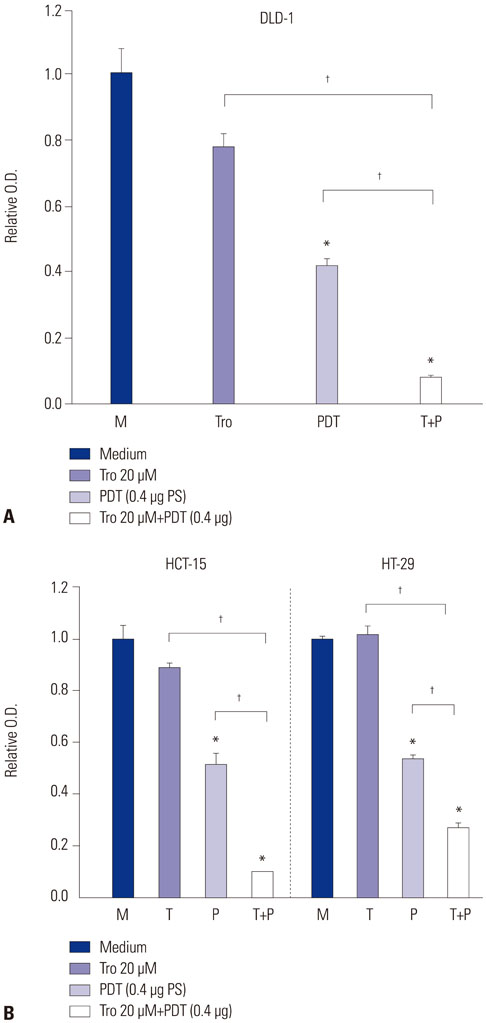
Treatment of troglitazone and PDT induced the growth retardation and cell death of DLD-1 cells in a dose-dependent manner, respectively. The combination treatment significantly suppressed cell growth and increased the apoptotic response of DLD-1 and resulted in apoptosis rather than necrosis, as shown by PI/annexin V staining and degradation of procaspase-3.
CONCLUSION
Conclusion: These results document the anti-proliferative and apoptotic activities of PDT in combination with the PPARγ ligand troglitazone and provide a strong rationale for testing the therapeutic potential of combination treatment in colon cancer.
Keyword
MeSH Terms
-
Antineoplastic Agents/pharmacology/therapeutic use
Apoptosis/*drug effects
Blotting, Western
Caspase 3
Cell Cycle/drug effects
Cell Line, Tumor
Cell Proliferation/*drug effects
Cell Survival/*drug effects
Chromans/*pharmacology
Colonic Neoplasms/metabolism/pathology
Dose-Response Relationship, Drug
Humans
PPAR gamma/metabolism/*pharmacology
*Photochemotherapy
Tetrazolium Salts
Thiazoles
Thiazolidinediones/*pharmacology/therapeutic use
Antineoplastic Agents
Chromans
PPAR gamma
Tetrazolium Salts
Thiazoles
Thiazolidinediones
Caspase 3
Figure
Cited by 1 articles
-
Parameters of Glucose and Lipid Metabolism Affect the Occurrence of Colorectal Adenomas Detected by Surveillance Colonoscopies
Nam Hee Kim, Jung Yul Suh, Jung Ho Park, Dong Il Park, Yong Kyun Cho, Chong Il Sohn, Kyuyong Choi, Yoon Suk Jung
Yonsei Med J. 2017;58(2):347-354. doi: 10.3349/ymj.2017.58.2.347.
Reference
-
1. Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst. 1998; 90:889–905.
Article2. Krosl G, Korbelik M, Krosl J, Dougherty GJ. Potentiation of photodynamic therapy-elicited antitumor response by localized treatment with granulocyte-macrophage colony-stimulating factor. Cancer Res. 1996; 56:3281–3286.3. Kim HR, Luo Y, Li G, Kessel D. Enhanced apoptotic response to photodynamic therapy after bcl-2 transfection. Cancer Res. 1999; 59:3429–3432.4. Usuda J, Okunaka T, Furukawa K, Tsuchida T, Kuroiwa Y, Ohe Y, et al. Increased cytotoxic effects of photodynamic therapy in IL-6 gene transfected cells via enhanced apoptosis. Int J Cancer. 2001; 93:475–480.
Article5. Vanden Heuvel JP. Peroxisome proliferator-activated receptors: a critical link among fatty acids, gene expression and carcinogenesis. J Nutr. 1999; 129:2S Suppl. 575S–580S.
Article6. Gampe RT Jr, Montana VG, Lambert MH, Miller AB, Bledsoe RK, Milburn MV, et al. Asymmetry in the PPARgamma/RXRalpha crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol Cell. 2000; 5:545–555.
Article7. Mueller E, Sarraf P, Tontonoz P, Evans RM, Martin KJ, Zhang M, et al. Terminal differentiation of human breast cancer through PPAR gamma. Mol Cell. 1998; 1:465–470.8. Badawi AF, Badr MZ. Expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-gamma and levels of prostaglandin E2 and 15-deoxy-delta12,14-prostaglandin J2 in human breast cancer and metastasis. Int J Cancer. 2003; 103:84–90.
Article9. Kim KY, Kim SS, Cheon HG. Differential anti-proliferative actions of peroxisome proliferator-activated receptor-gamma agonists in MCF-7 breast cancer cells. Biochem Pharmacol. 2006; 72:530–540.
Article10. DuBois RN, Gupta R, Brockman J, Reddy BS, Krakow SL, Lazar MA. The nuclear eicosanoid receptor, PPARgamma, is aberrantly expressed in colonic cancers. Carcinogenesis. 1998; 19:49–53.
Article11. Tontonoz P, Singer S, Forman BM, Sarraf P, Fletcher JA, Fletcher CD, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gamma and the retinoid X receptor. Proc Natl Acad Sci U S A. 1997; 94:237–241.
Article12. Chang TH, Szabo E. Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor gamma in non-small cell lung cancer. Cancer Res. 2000; 60:1129–1138.13. Han S, Wada RK, Sidell N. Differentiation of human neuroblastoma by phenylacetate is mediated by peroxisome proliferator-activated receptor gamma. Cancer Res. 2001; 61:3998–4002.14. Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, et al. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998; 4:1046–1052.
Article15. Avis I, Hong SH, Martinez A, Moody T, Choi YH, Trepel J, et al. Five-lipoxygenase inhibitors can mediate apoptosis in human breast cancer cell lines through complex eicosanoid interactions. FASEB J. 2001; 15:2007–2009.
Article16. Butler R, Mitchell SH, Tindall DJ, Young CY. Nonapoptotic cell death associated with S-phase arrest of prostate cancer cells via the peroxisome proliferator-activated receptor gamma ligand, 15-deoxy-delta12,14-prostaglandin J2. Cell Growth Differ. 2000; 11:49–61.17. Tsubouchi Y, Sano H, Kawahito Y, Mukai S, Yamada R, Kohno M, et al. Inhibition of human lung cancer cell growth by the peroxisome proliferator-activated receptor-gamma agonists through induction of apoptosis. Biochem Biophys Res Commun. 2000; 270:400–405.
Article18. Han S, Roman J. Peroxisome proliferator-activated receptor gamma: a novel target for cancer therapeutics? Anticancer Drugs. 2007; 18:237–244.
Article19. Göke R, Göke A, Göke B, Chen Y. Regulation of TRAIL-induced apoptosis by transcription factors. Cell Immunol. 2000; 201:77–82.
Article20. Göke R, Göke A, Göke B, El-Deiry WS, Chen Y. Pioglitazone inhibits growth of carcinoid cells and promotes TRAIL-induced apoptosis by induction of p21waf1/cip1. Digestion. 2001; 64:75–80.
Article21. Kitamura S, Miyazaki Y, Shinomura Y, Kondo S, Kanayama S, Matsuzawa Y. Peroxisome proliferator-activated receptor gamma induces growth arrest and differentiation markers of human colon cancer cells. Jpn J Cancer Res. 1999; 90:75–80.
Article22. Agarwal ML, Clay ME, Harvey EJ, Evans HH, Antunez AR, Oleinick NL. Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells. Cancer Res. 1991; 51:5993–5996.23. Lam M, Oleinick NL, Nieminen AL. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells. Reactive oxygen species and mitochondrial inner membrane permeabilization. J Biol Chem. 2001; 276:47379–47386.24. Luo Y, Chang CK, Kessel D. Rapid initiation of apoptosis by photodynamic therapy. Photochem Photobiol. 1996; 63:528–534.
Article25. Chiu SM, Oleinick NL. Dissociation of mitochondrial depolarization from cytochrome c release during apoptosis induced by photodynamic therapy. Br J Cancer. 2001; 84:1099–1106.
Article26. Granville DJ, Carthy CM, Jiang H, Shore GC, McManus BM, Hunt DW. Rapid cytochrome c release, activation of caspases 3, 6, 7 and 8 followed by Bap31 cleavage in HeLa cells treated with photodynamic therapy. FEBS Lett. 1998; 437:5–10.
Article27. Musser DA, Oseroff AR. The use of tetrazolium salts to determine sites of damage to the mitochondrial electron transport chain in intact cells following in vitro photodynamic therapy with Photofrin II. Photochem Photobiol. 1994; 59:621–626.
Article28. Matroule JY, Carthy CM, Granville DJ, Jolois O, Hunt DW, Piette J. Mechanism of colon cancer cell apoptosis mediated by pyropheophorbide-a methylester photosensitization. Oncogene. 2001; 20:4070–4084.
Article29. Chinetti G, Griglio S, Antonucci M, Torra IP, Delerive P, Majd Z, et al. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998; 273:25573–25580.
Article30. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998; 391:79–82.
Article31. Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, et al. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci U S A. 2000; 97:4844–4849.
Article32. Koga H, Sakisaka S, Harada M, Takagi T, Hanada S, Taniguchi E, et al. Involvement of p21(WAF1/Cip1), p27(Kip1), and p18(INK4c) in troglitazone-induced cell-cycle arrest in human hepatoma cell lines. Hepatology. 2001; 33:1087–1097.
Article33. Morrison RF, Farmer SR. Role of PPARgamma in regulating a cascade expression of cyclin-dependent kinase inhibitors, p18(INK4c) and p21(Waf1/Cip1), during adipogenesis. J Biol Chem. 1999; 274:17088–17097.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synergistic Apoptotic Effect of Combination Treatment with Troglitazone and COX-2 Inhibitor in Glioma Cells
- Kaempferol Synergistically Enhances Cisplatin-induced Apoptosis and Cell Cycle Arrest in Colon Cancer Cells
- Quantitative Assessment of Tumor Responses after Radiation Therapy in a DLD-1 Colon Cancer Mouse Model Using Serial Dynamic Contrast-Enhanced Magnetic Resonance Imaging
- Photodynamic Therapy induced Cell Death using ALA and 632nm Diode Laser in A549 Lung Cancer Cells
- Troglitazone Increases the Susceptibility to TRAIL-Induced Apoptosis in Thyroid Cancer Cell Lines