Investig Clin Urol.
2016 May;57(3):215-220. 10.4111/icu.2016.57.3.215.
Light-controlled relaxation of the rat penile corpus cavernosum using NOBL-1, a novel nitric oxide releaser
- Affiliations
-
- 1Graduate School of Pharmaceutical Sciences, Nagoya City University, Nagoya, Japan. kkimura@med.nagoya-cu.ac.jp
- 2Graduate School of Medical Sciences, Nagoya City University, Nagoya, Japan.
- KMID: 2426497
- DOI: http://doi.org/10.4111/icu.2016.57.3.215
Abstract
- PURPOSE
To investigate whether relaxation of the rat penile corpus cavernosum could be controlled with NOBL-1, a novel, light-controllable nitric oxide (NO) releaser.
MATERIALS AND METHODS
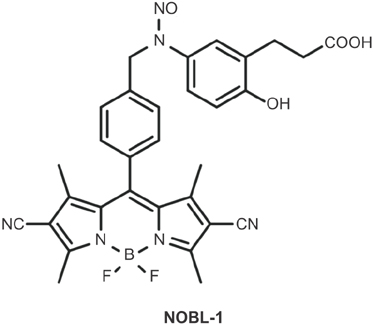
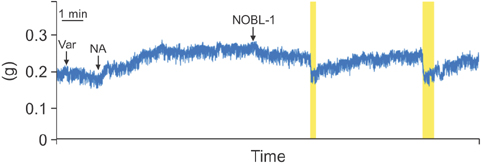
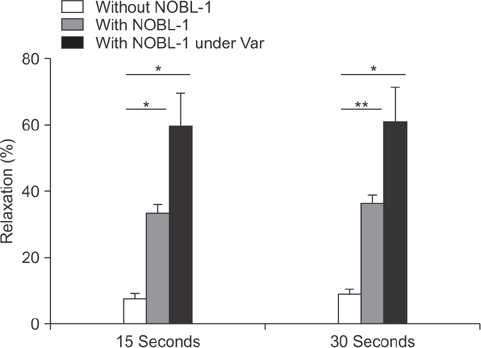
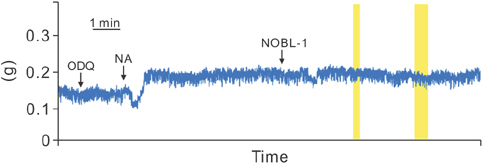
Fifteen-week-old male Wistar-ST rats were used. The penile corpus cavernosum was prepared and used in an isometric tension study. After noradrenaline (10-5 M) achieved precontraction, the penile corpus cavernosum was irradiated by light (470-500 nm) with and without NOBL-1 (10-6 M). In addition, we noted rats' responses to light with vardenafil (10-6 M), a phosphodiesterase-5 (PDE-5) inhibitor. Next, responses to light in the presence of a guanylate cyclase inhibitor, ODQ (1H-[1,2,4] oxadiazolo[4,3-a]quinoxalin-1-one) (10-5 M), were measured. All measurements were performed in pretreated L-NAME (10-4 M) conditions to inhibit endogenous NO production.
RESULTS
Corpus cavernosal smooth muscle, precontracted with noradrenaline, was unchanged by light irradiation in the absence of NOBL-1. However, in the presence of NOBL-1, corpus cavernosal smooth muscle, precontracted with noradrenaline, relaxed in response to light irradiation. After blue light irradiation ceased, tension returned. In addition, the light response was obviously enhanced in the presence of a PDE-5 inhibitor.
CONCLUSIONS
This study showed that rat corpus cavernosal smooth muscle relaxation can be light-controlled using NOBL-1, a novel, light sensitive NO releaser. Though further in vivo studies are needed to investigate possible usefulness, NOBL-1 may be prove to be a useful tool for erectile dysfunction therapy, specifically in the field of penile rehabilitation.
Keyword
MeSH Terms
-
Animals
Guanylate Cyclase/antagonists & inhibitors
Male
Muscle Relaxation/*drug effects/radiation effects
Muscle, Smooth/drug effects/radiation effects
Nitric Oxide/*physiology
Nitric Oxide Donors/*pharmacology
Oxadiazoles/pharmacology
Penis/*drug effects/physiology/radiation effects
Phosphodiesterase 5 Inhibitors/pharmacology
Photic Stimulation/*methods
Quinoxalines/pharmacology
Rats, Wistar
Vardenafil Dihydrochloride/pharmacology
Figure
Reference
-
1. Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992; 257:401–403.2. Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990; 170:843–850.3. Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, et al. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2002; 99:4061–4066.4. Hosogai N, Takakura S, Manda T, Mutoh S. Enzyme activities of the nitric oxide-cGMP pathway in corpus cavernosum isolated from middle-aged rats. Eur J Pharmacol. 2003; 473:65–70.5. Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995; 75:725–748.6. Soderling SH, Beavo JA. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol. 2000; 12:174–179.7. McMahon CG. Erectile dysfunction. Intern Med J. 2014; 44:18–26.8. Saenz de Tejada I, Goldstein I, Azadzoi K, Krane RJ, Cohen RA. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989; 320:1025–1030.9. Ieda N, Hotta Y, Miyata N, Kimura K, Nakagawa H. Photomanipulation of vasodilation with a blue-light-controllable nitric oxide releaser. J Am Chem Soc. 2014; 136:7085–7091.10. Nakagawa H, Hishikawa K, Eto K, Ieda N, Namikawa T, Kamada K, et al. Fine spatiotemporal control of nitric oxide release by infrared pulse-laser irradiation of a photolabile donor. ACS Chem Biol. 2013; 8:2493–2500.11. Namiki S, Kaneda F, Ikegami M, Arai T, Fujimori K, Asada S, et al. Bis-N-nitroso-caged nitric oxides: photochemistry and biological performance test by rat aorta vasorelaxation. Bioorg Med Chem. 1999; 7:1695–1702.12. Ueno T, Urano Y, Kojima H, Nagano T. Mechanism-based molecular design of highly selective fluorescence probes for nitrative stress. J Am Chem Soc. 2006; 128:10640–10641.13. Montorsi F, Guazzoni G, Strambi LF, Da Pozzo LF, Nava L, Barbieri L, et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. J Urol. 1997; 158:1408–1410.14. Kim JH, Lee SW. Current status of penile rehabilitation after radical prostatectomy. Korean J Urol. 2015; 56:99–108.15. Mulhall JP, Bella AJ, Briganti A, McCullough A, Brock G. Erectile function rehabilitation in the radical prostatectomy patient. J Sex Med. 2010; 7(4 Pt 2):1687–1698.16. Chung E, Brock G. Sexual rehabilitation and cancer survivorship: a state of art review of current literature and management strategies in male sexual dysfunction among prostate cancer survivors. J Sex Med. 2013; 10:Suppl 1. 102–111.17. Salonia A, Burnett AL, Graefen M, Hatzimouratidis K, Montorsi F, Mulhall JP, et al. Prevention and management of postprostatectomy sexual dysfunctions part 2: recovery and preservation of erectile function, sexual desire, and orgasmic function. Eur Urol. 2012; 62:273–286.18. Carrier S, Zvara P, Nunes L, Kour NW, Rehman J, Lue TF. Regeneration of nitric oxide synthase-containing nerves after cavernous nerve neurotomy in the rat. J Urol. 1995; 153:1722–1727.19. Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013; 381:153–165.20. Heidelbaugh JJ. Management of erectile dysfunction. Am Fam Physician. 2010; 81:305–312.21. Smith WB 2nd, McCaslin IR, Gokce A, Mandava SH, Trost L, Hellstrom WJ. PDE5 inhibitors: considerations for preference and long-term adherence. Int J Clin Pract. 2013; 67:768–780.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Nitric Oxide in the Relaxation of Canine Corpus Cavernosum Smooth Muscle
- Mechanism of Action of Endothelium-Dependent Relaxation Substances on Rabbit Corpus Cavernosum
- The Expression of eNOS and ET-1 in Corpus Cavernosum in Male Rat with Partial Bladder Outlet Obstruction
- Role of nitric oxide and vasoactive intestinal peptide in the relaxation of the smooth muscle of the rabbit corpus cavernosum
- Experimental evidence for endothelium dependent relaxation and neuronal nitric oxide in corpus cavernosum