Ann Lab Med.
2017 Nov;37(6):516-521. 10.3343/alm.2017.37.6.516.
A Unique Mutational Spectrum of MLC1 in Korean Patients With Megalencephalic Leukoencephalopathy With Subcortical Cysts: p.Ala275Asp Founder Mutation and Maternal Uniparental Disomy of Chromosome 22
- Affiliations
-
- 1Department of Pediatrics, Pediatric Clinical Neuroscience Center, Seoul National University Children's Hospital, Seoul National University College of Medicine, Seoul, Korea. prabbit7@snu.ac.kr
- 2Department of Laboratory Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. mwseong@snu.ac.kr
- 3Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 4Department of Pediatrics, Seoul National University Bundang Hospital, Seongnam, Korea.
- 5Department of Pediatrics, Seoul National University Boramae Medical Center, Seoul, Korea.
- 6Department of Pediatrics, Jeju National University Hospital, Jeju National University School of Medicine, Jeju, Korea.
- 7Department of Pediatrics, Pusan National University Children's Hospital, Pusan National University College of Medicine, Yangsan, Korea.
- KMID: 2425993
- DOI: http://doi.org/10.3343/alm.2017.37.6.516
Abstract
- BACKGROUND
Megalencephalic leukoencephalopathy with subcortical cysts (MLC) is a rare inherited disorder characterized by infantile-onset macrocephaly, slow neurologic deterioration, and seizures. Mutations in the causative gene, MLC1, are found in approximately 75% of patients and are inherited in an autosomal recessive manner. We analyzed MLC1 mutations in five unrelated Korean patients with MLC.
METHODS
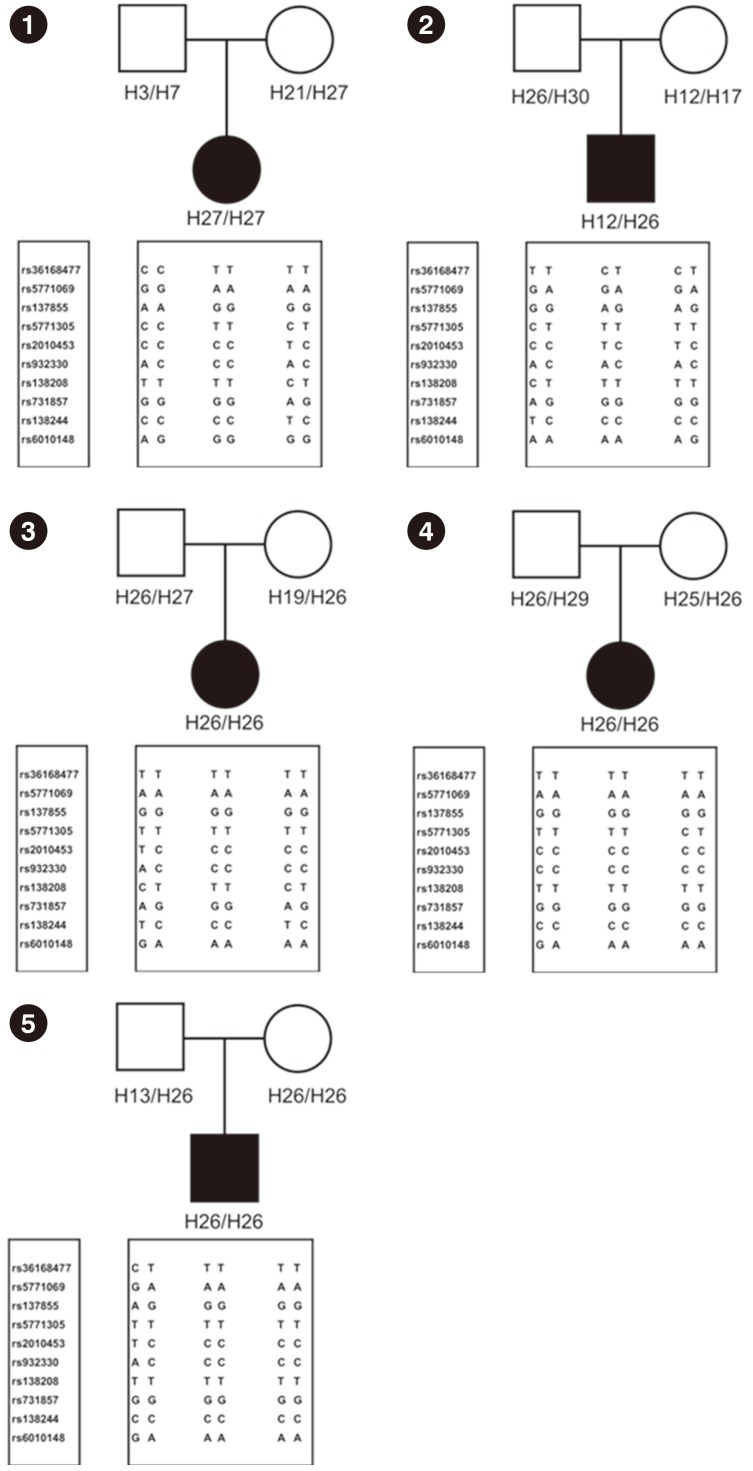
Direct Sanger sequencing was used to identify MLC1 mutations. A founder effect of the p.Ala275Asp variant was demonstrated by haplotype analysis using single-nucleotide polymorphic (SNP) markers. Multiple ligation-dependent probe amplification (MLPA) and comparative genomic hybridization plus SNP array were used to detect exonic deletions or uniparental disomy (UPD).
RESULTS
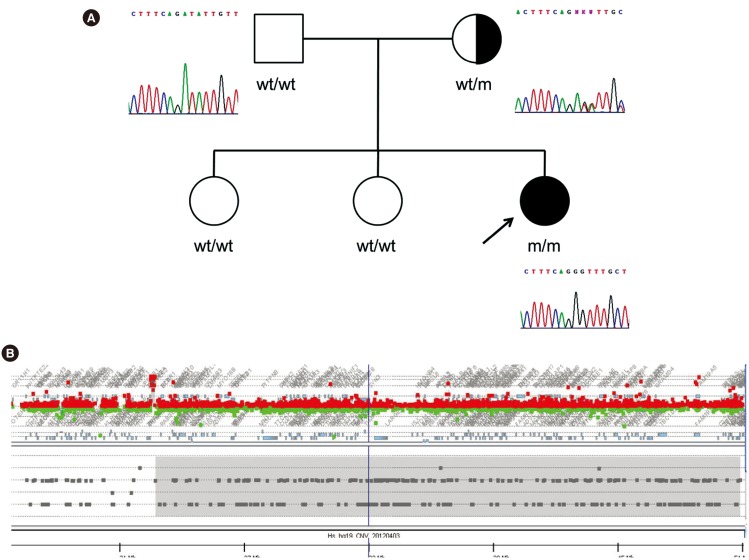
The most prevalent pathogenic variant was c.824C>A (p.Ala275Asp) found in 7/10 (70%) alleles. Two pathogenic frameshift variants were found: c.135delC (p.Cys46Alafs*12) and c.337_353delinsG (p.Ile113Glyfs*4). Haplotype analysis suggested that the Korean patients with MLC harbored a founder mutation in p.Ala275Asp. The p.(Ile113Glyfs*4) was identified in a homozygous state, and a family study revealed that only the mother was heterozygous for this variant. Further analysis of MLPA and SNP arrays for this patient demonstrated loss of heterozygosity of chromosome 22 without any deletion, indicating UPD. The maternal origin of both chromosomes 22 was demonstrated by haplotype analysis.
CONCLUSIONS
This study is the first to describe the mutational spectrum of Korean patients with MLC, demonstrating a founder effect of the p.Ala275Asp variant. This study also broadens our understanding of the mutational spectrum of MLC1 by demonstrating a homozygous p.(Ile113Glyfs*4) variant resulting from UPD of chromosome 22.
Keyword
MeSH Terms
Figure
Reference
-
1. van der Knaap MS, Barth PG, Stroink H, van Nieuwenhuizen O, Arts WF, Hoogenraad F, et al. Leukoencephalopathy with swelling and a discrepantly mild clinical course in eight children. Ann Neurol. 1995; 37:324–334. PMID: 7695231.2. Topcu M, Saatci I, Topcuoglu MA, Kose G, Kunak B. Megalencephaly and leukodystrophy with mild clinical course: a report on 12 new cases. Brain Dev. 1998; 20:142–153. PMID: 9628190.3. Topçu M, Gartioux C, Ribierre F, Yalçinkaya C, Tokus E, Oztekin N, et al. Vacuoliting megalencephalic leukoencephalopathy with subcortical cysts, mapped to chromosome 22qtel. Am J Hum Genet. 2000; 66:733–739. PMID: 10677334.4. Leegwater PA, Yuan BQ, van der Steen J, Mulders J, Könst AA, Boor PK, et al. Mutations of MLC1 (KIAA0027), encoding a putative membrane protein, cause megalencephalic leukoencephalopathy with subcortical cysts. Am J Hum Genet. 2001; 68:831–838. PMID: 11254442.5. van der Knaap MS, Boor I, Estévez R. Megalencephalic leukoencephalopathy with subcortical cysts: chronic white matter oedema due to a defect in brain ion and water homoeostasis. Lancet Neurol. 2012; 11:973–985. PMID: 23079554.6. López-Hernández T, Sirisi S, Capdevila-Nortes X, Montolio M, Fernández-Dueñas V, Scheper GC, et al. Molecular mechanisms of MLC1 and GLIALCAM mutations in megalencephalic leukoencephalopathy with subcortical cysts. Hum Mol Genet. 2011; 20:3266–3277. PMID: 21624973.7. Leegwater PA, Boor PK, Yuan BQ, van der Steen J, Visser A, Könst AA, et al. Identification of novel mutations in MLC1 responsible for megalencephalic leukoencephalopathy with subcortical cysts. Hum Genet. 2002; 110:279–283. PMID: 11935341.8. Ilja Boor PK, de Groot K, Mejaski-Bosnjak V, Brenner C, van der Knaap MS, Scheper GC, et al. Megalencephalic leukoencephalopathy with subcortical cysts: an update and extended mutation analysis of MLC1. Hum Mutat. 2006; 27:505–512. PMID: 16652334.9. Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN. The human gene mutation database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. 2014; 133:1–9. PMID: 24077912.10. Gorospe JR, Singhal BS, Kainu T, Wu F, Stephan D, Trent J, et al. Indian Agarwal megalencephalic leukodystrophy with cysts is caused by a common MLC1 mutation. Neurology. 2004; 62:878–882. PMID: 15037685.11. Ben-Zeev B, Levy-Nissenbaum E, Lahat H, Anikster Y, Shinar Y, Brand N, et al. Megalencephalic leukoencephalopathy with subcortical cysts; a founder effect in Israeli patients and a higher than expected carrier rate among Libyan Jews. Hum Genet. 2002; 111:214–218. PMID: 12189496.12. Mahmoud IG, Mahmoud M, Refaat M, Girgis M, Waked N, El Badawy A, et al. Clinical, neuroimaging, and genetic characteristics of megalencephalic leukoencephalopathy with subcortical cysts in Egyptian patients. Pediatr Neurol. 2014; 50:140–148. PMID: 24315536.13. Tsujino S, Kanazawa N, Yoneyama H, Shimono M, Kawakami A, Hatanaka Y, et al. A common mutation and a novel mutation in Japanese patients with van der Knaap disease. J Hum Genet. 2003; 48:605–608. PMID: 14615938.14. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424. PMID: 25741868.15. Schneider S, Roessli D, et al. Arlequin: a software for population genetics data analysis. User manual ver 2.000. Geneva: Genetics and Biometry Lab, Dept. of Anthropology, University of Geneva;2000.16. Stephens M, Scheet P. Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am J Hum Genet. 2005; 76:449–462. PMID: 15700229.17. Montagna G, Teijido O, Eymard-Pierre E, Muraki K, Cohen B, Loizzo A, et al. Vacuolating megalencephalic leukoencephalopathy with subcortical cysts: functional studies of novel variants in MLC1. Hum Mutat. 2006; 27:292.18. Shimada S, Shimojima K, Masuda T, Nakayama Y, Kohji T, Tsukamoto H, et al. MLC1 mutations in Japanese patients with megalencephalic leukoencephalopathy with subcortical cysts. Hum Genome Var. 2014; 1:14019. PMID: 27081509.19. Saijo H, Nakayama H, Ezoe T, Araki K, Sone S, Hamaguchi H, et al. A case of megalencephalic leukoencephalopathy with subcortical cysts (van der Knaap disease): molecular genetic study. Brain Dev. 2003; 25:362–366. PMID: 12850517.20. Ledbetter DH, Engel E. Uniparental disomy in humans: development of an imprinting map and its implications for prenatal diagnosis. Hum Mol Genet. 1995; 4:1757–1764. PMID: 8541876.21. Engel E, DeLozier-Blanchet CD. Uniparental disomy, isodisomy, and imprinting: probable effects in man and strategies for their detection. Am J Med Genet. 1991; 40:432–439. PMID: 1746607.22. Zlotogora J. Parents of children with autosomal recessive diseases are not always carriers of the respective mutant alleles. Hum Genet. 2004; 114:521–526. PMID: 15024643.23. Niida Y, Kuroda M, Mitani Y, Yokoi A, Ozaki M. Paternal uniparental isodisomy of chromosome 22 in a patient with metachromatic leukodystrophy. J Hum Genet. 2012; 57:687–690. PMID: 22854541.24. Huang YH, Tai CL, Lu YH, Wu TJ, Chen HD, Niu DM. Recessive congenital methemoglobinemia caused by a rare mechanism: maternal uniparental heterodisomy with segmental isodisomy of a chromosome 22. Blood Cells Mol Dis. 2012; 49:114–117. PMID: 22658170.25. Zhang P, Gao Z, Jiang Y, Wang J, Zhang F, Wang S, et al. Follow-up study of 25 Chinese children with PLA2G6-associated neurodegeneration. Eur J Neurol. 2013; 20:322–330. PMID: 22934738.26. Cao B, Yan H, Guo M, Xie H, Wu Y, Gu Q, et al. Ten novel mutations in Chinese patients with megalencephalic leukoencephalopathy with subcortical cysts and a long-term follow-up research. PLoS One. 2016; 11:e0157258. PMID: 27322623.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of maternal uniparental disomy of chromosome 20 detected by noninvasive prenatal test of 1,000 high-risk pregnancies
- A case of megalencephalic leukoencephalopathy with subcortical cysts
- Neonatal Silver-Russell syndrome assumed to result from maternal uniparental heterodisomy of chromosome 7
- A Case of Prader-Willi Syndrome with Microdeletion of Chromosome 15 q11-q13 Confirmed by FISH
- Clinical Characteristics of Cerebral Autosomal-Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy Patients with R544C Mutation Aged 90 or Older