Korean J Radiol.
2018 Feb;19(1):85-92. 10.3348/kjr.2018.19.1.85.
Magnetic Resonance Image Texture Analysis of the Periaqueductal Gray Matter in Episodic Migraine Patients without T2-Visible Lesions
- Affiliations
-
- 1Department of Radiology, Chinese PLA General Hospital, Beijing 100853, China. cjr.malin@vip.163.com
- 2Department of Neurology, Chinese PLA General Hospital, Beijing 100853, China.
- 3Department of Radiology, Hainan Branch of Chinese PLA General Hospital, Sanya 572013, China.
- KMID: 2425113
- DOI: http://doi.org/10.3348/kjr.2018.19.1.85
Abstract
OBJECTIVE
The periaqueductal gray matter (PAG), a small midbrain structure, presents dysfunction in migraine. However, the precise neurological mechanism is still not well understood. Herein, the aim of this study was to investigate the texture characteristics of altered PAG in episodic migraine (EM) patients based on high resolution brain structural magnetic resonance (MR) images.
MATERIALS AND METHODS
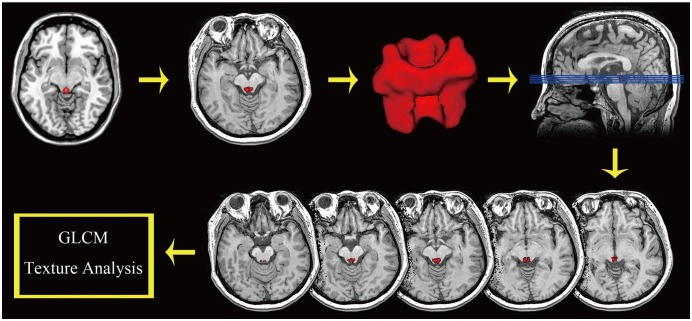
The brain structural MR images were obtained from 18 normal controls (NC), 18 EM patients and 16 chronic migraine (CM) patients using a 3T MR system. A PAG template was created using the International Consortium Brain Mapping 152 gray matter model, and the individual PAG segment was developed by applying the deformation field from the structural image segment to the PAG template. A grey level co-occurrence matrix was used to calculate the texture parameters including the angular second moment (ASM), contrast, correlation, inverse difference moment (IDM) and entropy.
RESULTS
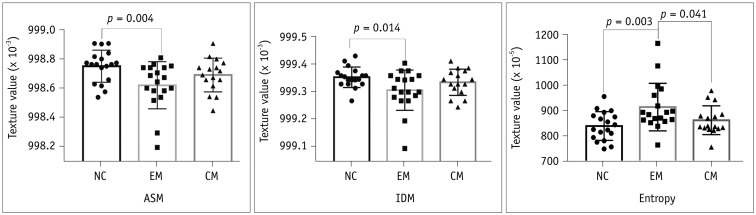
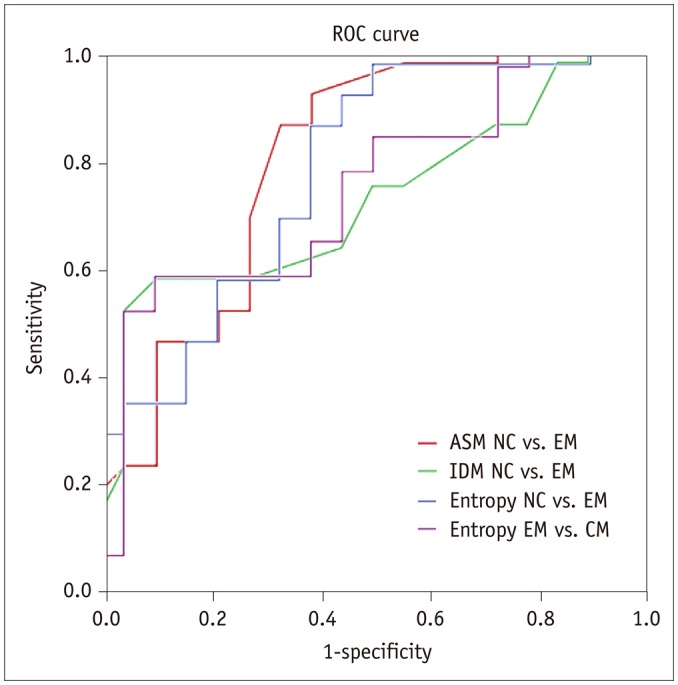
There was a significant difference for ASM, IDM and entropy in the EM group (998.629 ± 0.162 × 10−3, 999.311 ± 0.073 × 10−3, 916.354 ± 0.947 × 10−5) compared to that found in the NC group (998.760 ± 0.110 × 10−3, 999.358 ± 0.037 × 10−3 and 841.198 ± 0.575 × 10−5) (p < 0.05). The entropy was significantly lower among the patients with CM (864.116 ± 0.571 × 10−5) than that found among patients with EM (p < 0.05). The area under the receiver operating characteristic curve was 0.776 and 0.750 for ASM and entropy in the distinction of the EM from NC groups, respectively. ASM was negatively related to disease duration (DD) and the Migraine Disability Assessment Scale (MIDAS) scores in the EM group, and entropy was positively related to DD and MIDAS in the EM group (p < 0.05).
CONCLUSION
The present study identified altered MR image texture characteristics of the PAG in EM. The identified texture characteristics could be considered as imaging biomarkers for EM.
Keyword
MeSH Terms
Figure
Reference
-
1. Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001; 41:629–637. PMID: 11554950.
Article2. Smith GS, Savery D, Marden C, López Costa JJ, Averill S, Priestley JV, et al. Distribution of messenger RNAs encoding enkephalin, substance P, somatostatin, galanin, vasoactive intestinal polypeptide, neuropeptide Y, and calcitonin gene-related peptide in the midbrain periaqueductal grey in the rat. J Comp Neurol. 1994; 350:23–40. PMID: 7860799.
Article3. Kruit MC, Launer LJ, Overbosch J, van Buchem MA, Ferrari MD. Iron accumulation in deep brain nuclei in migraine: a population-based magnetic resonance imaging study. Cephalalgia. 2009; 29:351–359. PMID: 19025553.
Article4. Tepper SJ, Lowe MJ, Beall E, Phillips MD, Liu K, Stillman MJ, et al. Iron deposition in pain-regulatory nuclei in episodic migraine and chronic daily headache by MRI. Headache. 2012; 52:236–243. PMID: 22188387.
Article5. Raskin NH, Hosobuchi Y, Lamb S. Headache may arise from perturbation of brain. Headache. 1987; 27:416–420. PMID: 3667258.
Article6. Gee JR, Chang J, Dublin AB, Vijayan N. The association of brainstem lesions with migraine-like headache: an imaging study of multiple sclerosis. Headache. 2005; 45:670–677. PMID: 15953299.
Article7. Haas DC, Kent PF, Friedman DI. Headache caused by a single lesion of multiple sclerosis in the periaqueductal gray area. Headache. 1993; 33:452–455. PMID: 8262789.
Article8. Lin GY, Wang CW, Chiang TT, Peng GS, Yang FC. Multiple sclerosis presenting initially with a worsening of migraine symptoms. J Headache Pain. 2013; 14:70. PMID: 23937696.
Article9. Tortorella P, Rocca MA, Colombo B, Annovazzi P, Comi G, Filippi M. Assessment of MRI abnormalities of the brainstem from patients with migraine and multiple sclerosis. J Neurol Sci. 2006; 244:137–141. PMID: 16530789.
Article10. Fragoso YD, Brooks JB. Two cases of lesions in brainstem in multiple sclerosis and refractory migraine. Headache. 2007; 47:852–854. PMID: 17578534.
Article11. Wang Y, Wang XS. Migraine-like headache from an infarction in the periaqueductal gray area of the midbrain. Pain Med. 2013; 14:948–949. PMID: 23565756.
Article12. Chen Z, Chen X, Liu M, Liu S, Ma L, Yu S. Nonspecific periaqueductal gray lesions on T2WI in episodic migraine. J Headache Pain. 2016; 17:101. PMID: 27796733.
Article13. Chen Z, Chen X, Liu M, Liu S, Ma L, Yu S, et al. Disrupted functional connectivity of periaqueductal gray subregions in episodic migraine. J Headache Pain. 2017; 18:36. PMID: 28321594.
Article14. Herlidou-Meˆme S, Constans JM, Carsin B, Olivie D, Eliat PA, Nadal-Desbarats L, et al. MRI texture analysis on texture test objects, normal brain and intracranial tumors. Magn Reson Imaging. 2003; 21:989–993. PMID: 14684201.15. Nachimuthu DS, Baladhandapani A. Multidimensional texture characterization: on analysis for brain tumor tissues using MRS and MRI. J Digit Imaging. 2014; 27:496–506. PMID: 24496552.
Article16. Mahmoud-Ghoneim D, Toussaint G, Constans JM, de Certaines JD. Three dimensional texture analysis in MRI: a preliminary evaluation in gliomas. Magn Reson Imaging. 2003; 21:983–987. PMID: 14684200.
Article17. de Oliveira MS, Betting LE, Mory SB, Cendes F, Castellano G. Texture analysis of magnetic resonance images of patients with juvenile myoclonic epilepsy. Epilepsy Behav. 2013; 27:22–28. PMID: 23357730.
Article18. Caselato GR, Kobayashi E, Bonilha L, Castellano G, Rigas AH, Li LM, et al. Hippocampal texture analysis in patients with familial mesial temporal lobe epilepsy. Arq Neuropsiquiatr. 2003; 61(Suppl 1):83–87.19. Certaines JDD, Larcher T, Duda D, Azzabou N, Eliat PA, Escudero LM, et al. Application of texture analysis to muscle MRI: 1-What kind of information should be expected from texture analysis? EPJ Nonlinear Biomed Phys. 2015; 3:1–14.
Article20. Chang CW, Ho CC, Chen JH. ADHD classification by a texture analysis of anatomical brain MRI data. Front Syst Neurosci. 2012; 6:66. PMID: 23024630.
Article21. de Oliveira MS, Balthazar ML, D'Abreu A, Yasuda CL, Damasceno BP, Cendes F, et al. MR imaging texture analysis of the corpus callosum and thalamus in amnestic mild cognitive impairment and mild Alzheimer disease. AJNR Am J Neuroradiol. 2011; 32:60–66. PMID: 20966061.
Article22. Haralick RM, Shanmugam K, Dinstein IH. Textural Features for Image Classification. IEEE Trans Syst Man Cybern. 1973; smc-3:610–621.
Article23. Rajković N, Kolarević D, Kanjer K, Milošević NT, Nikolić-Vukosavljević D, Radulovic M. Comparison of Monofractal, Multifractal and gray level Co-occurrence matrix algorithms in analysis of Breast tumor microscopic images for prognosis of distant metastasis risk. Biomed Microdevices. 2016; 18:83. PMID: 27549346.
Article24. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013; 33:629–680. PMID: 23771276.25. Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord. 1988; 14:61–68. PMID: 2963053.
Article26. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967; 6:278–296. PMID: 6080235.
Article27. Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000; 11:805–821. PMID: 10860804.
Article28. Walker RF, Jackway P, Longstaff ID. Improving co-occurrence matrix feature discrimination. In : DICTA '95, 3rd Conference on Digital Image Computing: Techniques and Application; 1995 December 6-8; p. 643–648.29. Mohanaiah P, Sathyanarayana P, Gurukumar L. Image texture feature extraction using GLCM approach. Int J Sci Res Publ. 2013; 3:1–5.30. Benarroch EE. Periaqueductal gray: an interface for behavioral control. Neurology. 2012; 78:210–217. PMID: 22249496.
Article31. An X, Bandler R, Ongür D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998; 401:455–479. PMID: 9826273.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion Weighted Magnetic Resonance Imaging in a Patient with Acute Wernicke Encephalopathy
- A Case of Wernicke's Encephalopathy in a Patient with Multiple System Atrophy
- Perinatal Hypoxic-lschemic Brain Injury: MR Findings
- MRI Findings in Wernicke's Encephalopathy with Hyperemesis Gravidarum
- Comparison of FSE and EPI with Brain MR Imaging