Cancer Res Treat.
2018 Oct;50(4):1458-1461. 10.4143/crt.2017.529.
Pembrolizumab for Refractory Metastatic Myxofibrosarcoma: A Case Report
- Affiliations
-
- 1Divisions of Hematology and Oncology, Department of Internal Medicine, Institute of Health Science, Gyeongsang National University Hospital, Gyeongsang National University College of Medicine, Jinju, Korea. brightree24@gmail.com
- 2Divisions of Cardiology, Department of Internal Medicine, Institute of Health Science, Gyeongsang National University Hospital, Gyeongsang National University College of Medicine, Jinju, Korea.
- KMID: 2424816
- DOI: http://doi.org/10.4143/crt.2017.529
Abstract
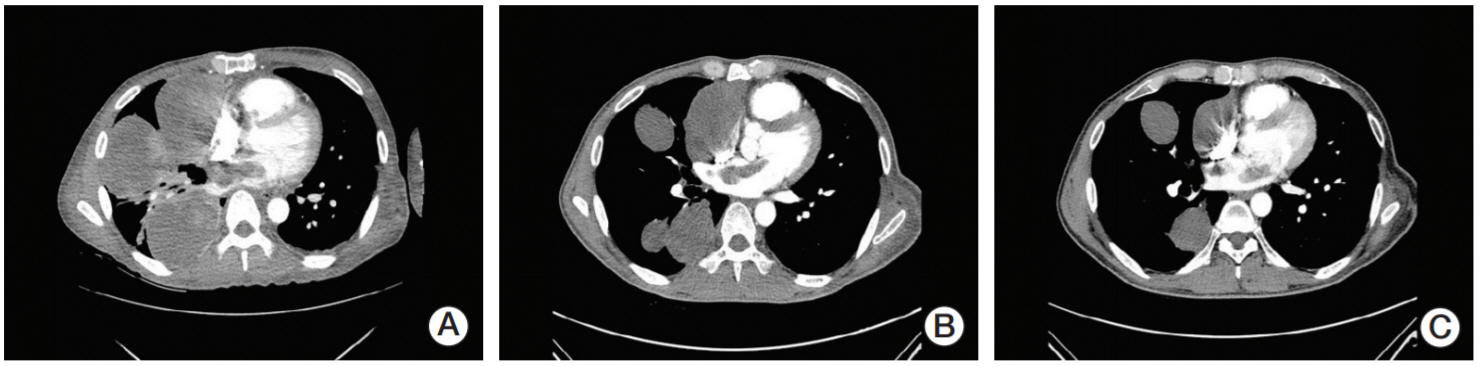
- Myxofibrosarcoma is a rare tumor, refractory to cytotoxic chemotherapy and radiotherapy. Pembrolizumab is an innovative immunotherapy drug consisting of programmed death receptor ligand 1 antibody proven to be useful for numerous types of cancer cells. A patient had been diagnosed with metastatic myxofibrosarcoma, refractory to radiotherapy and conventional cytotoxic chemotherapy. The patient achieved a partial response during palliative chemotherapy with pembrolizumab for 14 cycles. To the best of our knowledge, this is the first case report demonstrating the efficacy of pembrolizumab for refractory myxofibrosarcoma.
Keyword
Figure
Reference
-
References
1. Lazaros GA, Matsakas EP, Madas JS, Toli DI, Nikas DJ, Kershaw MA, et al. Primary myxofibrosarcoma of the left atrium: case report and review of the literature. Angiology. 2008; 59:632–5.
Article2. McMillan RR, Sima CS, Moraco NH, Rusch VW, Huang J. Recurrence patterns after resection of soft tissue sarcomas of the chest wall. Ann Thorac Surg. 2013; 96:1223–8.
Article3. Kikuta K, Kubota D, Yoshida A, Suzuki Y, Morioka H, Toyama Y, et al. An analysis of factors related to recurrence of myxofibrosarcoma. Jpn J Clin Oncol. 2013; 43:1093–104.
Article4. Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016; 17:976–83.
Article5. Zschabitz S, Lasitschka F, Hadaschik B, Hofheinz RD, Jentsch-Ullrich K, Gruner M, et al. Response to anti-programmed cell death protein-1 antibodies in men treated for platinum refractory germ cell cancer relapsed after high-dose chemotherapy and stem cell transplantation. Eur J Cancer. 2017; 76:1–7.6. Rexer H, Steiner T, Grunwald V. First-line therapy in advanced renal cell carcinoma: a randomized phase II study to examine early switch of tyrosine kinase inhibitors to nivolumab compared to continued tyrosine kinase inhibitor therapy in patients with advanced or metastatic renal cell carcinoma and stable disease after three months of treatment (NIVOSWITCH)-AN 38/15 of the AUO. Urologe A. 2017; 56:509–11.7. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017; 376:1015–26.
Article8. Fletcher CD, Unni KK, Mertens F. World Health Organization classification of tumours: pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press;2002. p. 102–3.9. Nascimento AF, Bertoni F, Fletcher CD. Epithelioid variant of myxofibrosarcoma: expanding the clinicomorphologic spectrum of myxofibrosarcoma in a series of 17 cases. Am J Surg Pathol. 2007; 31:99–105.
Article10. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014; 515:568–71.
Article11. Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin Cancer Res. 2016; 22:5487–96.
Article12. Zhu Z, Jin Z, Zhang M, Tang Y, Yang G, Yuan X, et al. Prognostic value of programmed death-ligand 1 in sarcoma: a meta-analysis. Oncotarget. 2017; 8:59570–80.
Article13. Kosemehmetoglu K, Ozogul E, Babaoglu B, Tezel GG, Gedikoglu G. Programmed death ligand 1 (PD-L1) expression in malignant mesenchymal tumors. Turk Patoloji Derg. 2017; 1:192–7.
Article14. Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017; 18:1493–501.
Article15. D’Angelo SP, Mahoney MR, Van Tine BA, Atkins JN, Milhem MM, Tap WD, et al. A multi-center phase II study of nivolumab +/– ipilimumab for patients with metastatic sarcoma (Alliance A091401). J Clin Oncol. 2017; 35 Suppl:Abstr 11007.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Complete Remission after Pseudoprogression in Refractory Classical Hodgkin Lymphoma Treated with Pembrolizumab
- Myxofibrosarcoma of the Chest Wall
- A Case of Myxofibrosarcoma in the Cheek
- Exudative Retinal Detachment after Pembrolizumab Treatment in Metastatic Cutaneous Melanoma
- A Case of Pembrolizumab-Induced Toxic Epidermal Necrolysis