J Clin Neurol.
2018 Oct;14(4):555-565. 10.3988/jcn.2018.14.4.555.
Interarm Blood Pressure Difference is Associated with Early Neurological Deterioration, Poor Short-Term Functional Outcome, and Mortality in Noncardioembolic Stroke Patients
- Affiliations
-
- 1Department of Neurology, College of Medicine, Ewha Woman's University, Seoul, Korea. knstar@ewha.ac.kr
- 2Department of Neurology, College of Medicine, Korea University Guro Hospital, Seoul, Korea.
- 3Department of Neurology, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 4Department of Biostatistics, Ewha Institute of Convergency Medicine, Seoul, Korea.
- KMID: 2424186
- DOI: http://doi.org/10.3988/jcn.2018.14.4.555
Abstract
- BACKGROUND AND PURPOSE
Interarm differences in the systolic and diastolic blood pressures (IASBD and IADBD, respectively) are found in various populations, including stroke patients, but their significance for stroke outcomes has rarely been reported. We aimed to determine the associations of IASBD and IADBD with early neurological deterioration (END), functional outcome, and mortality.
METHODS
This study included 1,008 consecutive noncardioembolic cerebral infarction patients who were admitted within 24 hours of onset and had automatic measurements of blood pressures in the bilateral arms. END was assessed within 72 hours of stroke onset according to predefined criteria. A poor functional outcome was defined as a score on the modified Rankin Scale ≥3 at 3 months after the index stroke. All-cause mortality was also investigated during a median follow-up of 24 months. The absolute difference of blood pressure measurements in both arms were used to define IASBD and IADBD.
RESULTS
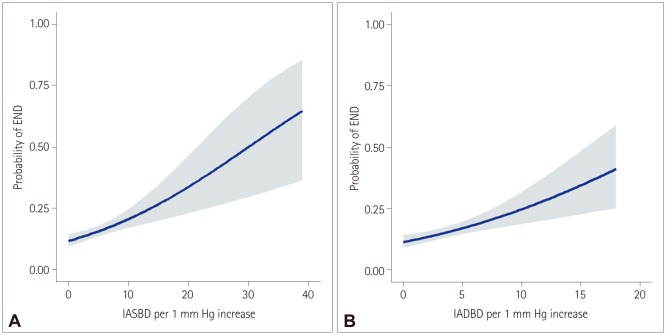
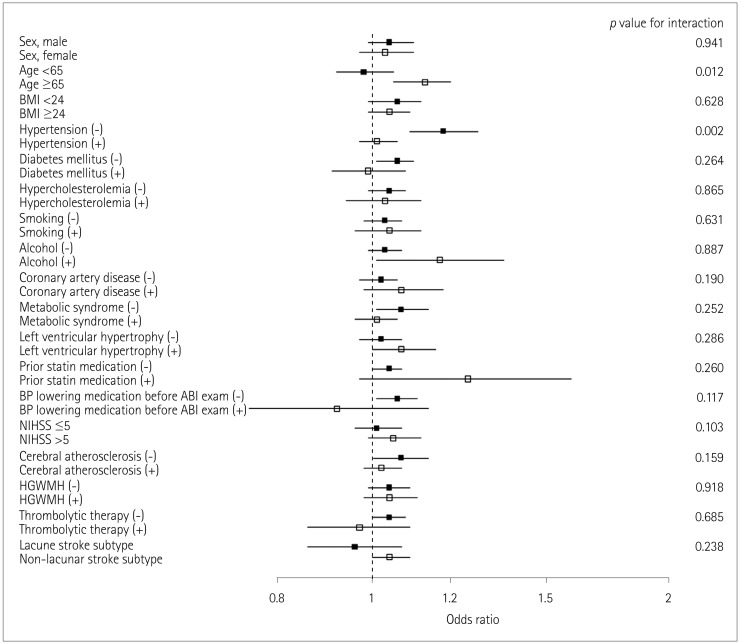
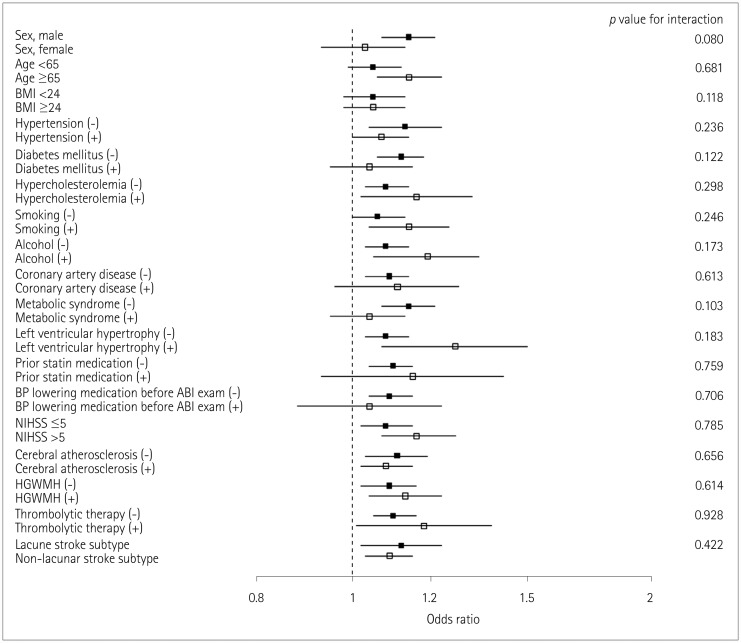
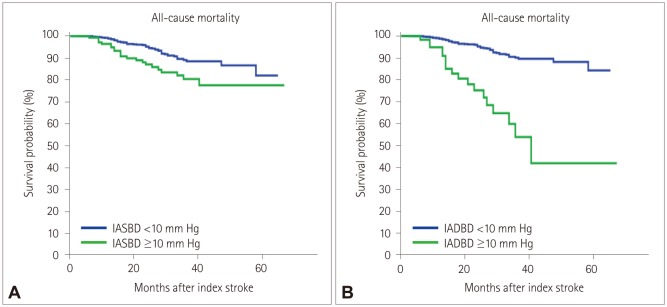
END occurred in 15.3% (155/1,008) of the patients. A multivariate analysis including sex, age, and variables for which the p value was < 0.1 in a univariate analysis revealed that IASBD ≥10 mm Hg was significantly associated with END [odds ratio (OR)=1.75, 95% CI=1.02-3.01]. IADBD ≥10 mm Hg was also related to END (OR=3.11, 95% CI=1.61-5.99). Moreover, having both IASBD ≥10 mm Hg and IADBD ≥10 mm Hg was related to a poor functional outcome (OR=2.67, 95% CI=1.36-5.35) and mortality (hazard ratio=7.67, 95% CI=3.76-12.83) even after adjusting for END.
CONCLUSIONS
This study suggests that an interarm blood pressure difference of ≥10 mm Hg could be a useful indicator for the risks of END, poor functional outcome, and mortality.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Plasma Fibroblast Growth Factor 23 Concentration Is Associated with Intracranial Cerebral Atherosclerosis in Acute Ischemic Stroke Patients
Yoonkyung Chang, Jinkwon Kim, Ho Geol Woo, Dong-Ryeol Ryu, Hyung Jung Oh, Tae-Jin Song
J Clin Neurol. 2020;16(1):29-36. doi: 10.3988/jcn.2020.16.1.29.Interarm Blood Pressure Difference has Various Associations with the Presence and Burden of Cerebral Small-Vessel Diseases in Noncardioembolic Stroke Patients
Yoonkyung Chang, Seung Ah Lee, Sue Hyun Lee, Eun Hye Lee, Yong-Jae Kim, Tae-Jin Song
J Clin Neurol. 2019;15(2):159-167. doi: 10.3988/jcn.2019.15.2.159.
Reference
-
1. Weimar C, Mieck T, Buchthal J, Ehrenfeld CE, Schmid E, Diener HC, et al. Neurologic worsening during the acute phase of ischemic stroke. Arch Neurol. 2005; 62:393–397. PMID: 15767504.
Article2. Thanvi B, Treadwell S, Robinson T. Early neurological deterioration in acute ischaemic stroke: predictors, mechanisms and management. Postgrad Med J. 2008; 84:412–417. PMID: 18832401.
Article3. Ois A, Martinez-Rodriguez JE, Munteis E, Gomis M, Rodríguez-Campello A, Jimenez-Conde J, et al. Steno-occlusive arterial disease and early neurological deterioration in acute ischemic stroke. Cerebrovasc Dis. 2008; 25:151–156. PMID: 18212520.
Article4. Dávalos A, Toni D, Iweins F, Lesaffre E, Bastianello S, Castillo J. Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke. 1999; 30:2631–2636. PMID: 10582989.5. Seners P, Turc G, Oppenheim C, Baron JC. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. 2015; 86:87–94. PMID: 24970907.6. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013; 31:1281–1357. PMID: 23817082.7. Clark CE, Taylor RS, Shore AC, Campbell JL. Prevalence of systolic inter-arm differences in blood pressure for different primary care populations: systematic review and meta-analysis. Br J Gen Pract. 2016; 66:e838–e847. PMID: 27789511.
Article8. Chang Y, Choi GS, Lim SM, Kim YJ, Song TJ. Interarm systolic and diastolic blood pressure difference is diversely associated with cerebral atherosclerosis in noncardioembolic stroke patients. Am J Hypertens. 2017; 31:35–42. PMID: 28985258.
Article9. Kim J, Song TJ, Song D, Lee HS, Nam CM, Nam HS, et al. Interarm blood pressure difference and mortality in patients with acute ischemic stroke. Neurology. 2013; 80:1457–1464. PMID: 23516316.
Article10. Clark CE, Aboyans V. Interarm blood pressure difference: more than an epiphenomenon. Nephrol Dial Transplant. 2015; 30:695–697. PMID: 25883198.
Article11. Roquer J, Ois A, Rodríguez-Campello A, Gomis M, Munteis E, Jiménez-Conde J, et al. Atherosclerotic burden and early mortality in acute ischemic stroke. Arch Neurol. 2007; 64:699–704. PMID: 17502469.
Article12. Song TJ, Cho HJ, Chang Y, Choi K, Jung AR, Youn M, et al. Low plasma proportion of omega 3-polyunsaturated fatty acids predicts poor outcome in acute non-cardiogenic ischemic stroke patients. J Stroke. 2015; 17:168–176. PMID: 26060804.
Article13. Stergiou GS, Kollias A, Destounis A, Tzamouranis D. Automated blood pressure measurement in atrial fibrillation: a systematic review and meta-analysis. J Hypertens. 2012; 30:2074–2082. PMID: 22914573.14. Motobe K, Tomiyama H, Koji Y, Yambe M, Gulinisa Z, Arai T, et al. Cut-off value of the ankle-brachial pressure index at which the accuracy of brachial-ankle pulse wave velocity measurement is diminished. Circ J. 2005; 69:55–60. PMID: 15635203.
Article15. Song TJ, Chang Y, Shin MJ, Heo JH, Kim YJ. Low levels of plasma omega 3-polyunsaturated fatty acids are associated with cerebral small vessel diseases in acute ischemic stroke patients. Nutr Res. 2015; 35:368–374. PMID: 25921638.
Article16. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41. PMID: 7678184.
Article17. Song TJ, Kim YD, Yoo J, Kim J, Chang HJ, Hong GR, et al. Association between aortic atheroma and cerebral small vessel disease in patients with ischemic stroke. J Stroke. 2016; 18:312–320. PMID: 27488980.
Article18. Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000; 21:643–646. PMID: 10782772.19. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991; 325:445–453. PMID: 1852179.
Article20. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987; 149:351–356. PMID: 3496763.
Article21. Chung JW, Kim N, Kang J, Park SH, Kim WJ, Ko Y, et al. Blood pressure variability and the development of early neurological deterioration following acute ischemic stroke. J Hypertens. 2015; 33:2099–2106. PMID: 26237556.
Article22. Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004; 62:569–573. PMID: 14981172.
Article23. Kim HC, Choi DP, Ahn SV, Nam CM, Suh I. Six-year survival and causes of death among stroke patients in Korea. Neuroepidemiology. 2009; 32:94–100. PMID: 19039241.
Article24. Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res. 2002; 25:359–364. PMID: 12135313.
Article25. Verberk WJ, Kessels AG, Thien T. Blood pressure measurement method and inter-arm differences: a meta-analysis. Am J Hypertens. 2011; 24:1201–1208. PMID: 21776035.
Article26. Clark CE, Taylor RS, Shore AC, Ukoumunne OC, Campbell JL. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012; 379:905–914. PMID: 22293369.
Article27. Aboyans V, Criqui MH, McDermott MM, Allison MA, Denenberg JO, Shadman R, et al. The vital prognosis of subclavian stenosis. J Am Coll Cardiol. 2007; 49:1540–1545. PMID: 17418292.
Article28. Clark CE, Taylor RS, Shore AC, Campbell JL. The difference in blood pressure readings between arms and survival: primary care cohort study. BMJ. 2012; 344:e1327. PMID: 22433975.
Article29. Agarwal R, Bunaye Z, Bekele DM. Prognostic significance of between-arm blood pressure differences. Hypertension. 2008; 51:657–662. PMID: 18212263.
Article30. Weinberg I, Gona P, O'Donnell CJ, Jaff MR, Murabito JM. The systolic blood pressure difference between arms and cardiovascular disease in the Framingham Heart Study. Am J Med. 2014; 127:209–215. PMID: 24287007.
Article31. Lee SJ, Lee DG. Distribution of atherosclerotic stenosis determining early neurologic deterioration in acute ischemic stroke. PLoS One. 2017; 12:e0185314. PMID: 28945817.
Article32. Spannella F, Giulietti F, Fedecostante M, Ricci M, Balietti P, Cocci G, et al. Interarm blood pressure differences predict target organ damage in type 2 diabetes. J Clin Hypertens (Greenwich). 2017; 19:472–478. PMID: 28026096.
Article33. Ochoa VM, Yeghiazarians Y. Subclavian artery stenosis: a review for the vascular medicine practitioner. Vasc Med. 2011; 16:29–34. PMID: 21078767.
Article34. Huang YC, Tsai YH, Lee JD, Weng HH, Lin LC, Lin YH, et al. Hemodynamic factors may play a critical role in neurological deterioration occurring within 72 hrs after lacunar stroke. PLoS One. 2014; 9:e108395. PMID: 25340713.
Article35. Hu W, Li J, Su H, Wang J, Xu J, Liu Y, et al. The inter-arm diastolic blood pressure difference induced by one arm ischemia: a new approach to assess vascular endothelia function. PLoS One. 2014; 9:e84765. PMID: 24454748.
Article36. Yi X, Han Z, Zhou Q, Lin J, Liu P. 20-Hydroxyeicosatetraenoic acid as a predictor of neurological deterioration in acute minor ischemic stroke. Stroke. 2016; 47:3045–3047. PMID: 27834744.
Article37. Canepa M, Milaneschi Y, Ameri P, AlGhatrif M, Leoncini G, Spallarossa P, et al. Relationship between inter-arm difference in systolic blood pressure and arterial stiffness in community-dwelling older adults. J Clin Hypertens (Greenwich). 2013; 15:880–887. PMID: 24299691.
Article38. Saji N, Kimura K, Kawarai T, Shimizu H, Kita Y. Arterial stiffness and progressive neurological deficit in patients with acute deep subcortical infarction. Stroke. 2012; 43:3088–3090. PMID: 22949476.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interarm Blood Pressure Difference has Various Associations with the Presence and Burden of Cerebral Small-Vessel Diseases in Noncardioembolic Stroke Patients
- Induced Hypertensive Therapy in an Acute Ischemic Stroke Patient with Early Neurological Deterioration
- Long-Term Mortality According to the Characteristics of Early Neurological Deterioration in Ischemic Stroke Patients
- A Low Baseline Glomerular Filtration Rate Predicts Poor Clinical Outcome at 3 Months after Acute Ischemic Stroke
- The Association between Neurological Prognosis and Serum Glucose Level by Stroke Subtype in Acute Ischemic Stroke