Korean J Orthod.
2018 Sep;48(5):339-345. 10.4041/kjod.2018.48.5.339.
Effects of continuous force application for extrusive tipping movement on periapical root resorption in the rat mandibular first molar
- Affiliations
-
- 1Department of Orthodontic Science, Division of Oral Health Sciences, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan. y.matsumoto.orts@tmd.ac.jp
- 2Private Practice, Bangkok, Thailand.
- KMID: 2421319
- DOI: http://doi.org/10.4041/kjod.2018.48.5.339
Abstract
OBJECTIVE
The purpose of this study was to clarify the effects of continuous force application for extrusive tipping movement and occlusal interference on periapical root resorption in the rat mandibular first molar.
METHODS
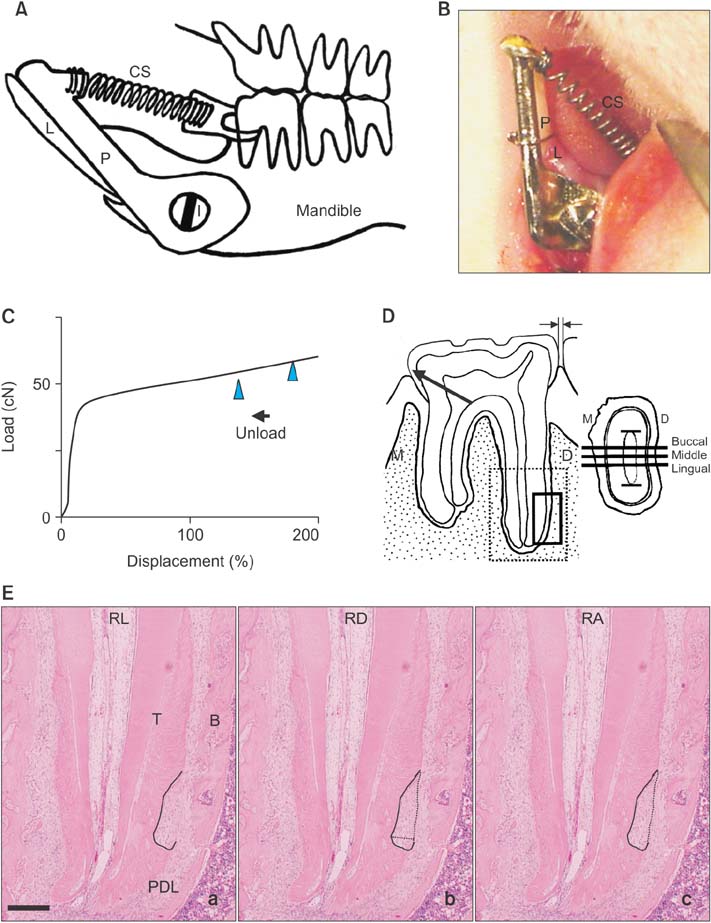
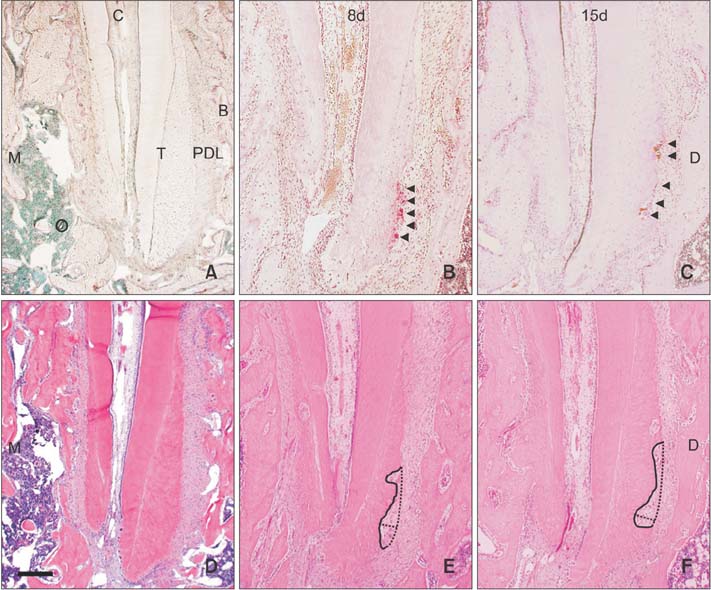
We constructed an appliance comprising a titanium screw implant with a cobalt-chromium post as the anchorage unit and a nickel-titanium closed coil spring (50 cN) as the active unit. Force was applied on the mandibular left first molar of rats for 8 (n = 10) and 15 days (n = 10; experimental groups), with the tooth in occlusion. Five rats were included as a non-treated control group to examine the body effect of the appliance. Active root resorption lacunae, identified using tartrate-resistant acid phosphatase, were evaluated in terms of the length, depth, and area.
RESULTS
The rat mandibular first molars were mesially tipped and extruded in the occlusal direction. This mesio-occlusal tipping movement and occlusion resulted in the formation of a compression zone and active root resorption lacunae in the distoapical third of the distal roots. However, there was no significant difference in the amount of root resorption between the two experimental groups. The control group did not exhibit any active root resorption lacunae.
CONCLUSIONS
Periapical root resorption was induced by continuous extrusive tipping force and occlusal interference in rat mandibular molars. These data suggest that we orthodontists had better take care not to induce occlusal interference during our orthodontic treatment.
MeSH Terms
Figure
Reference
-
1. Roscoe MG, Meira JB, Cattaneo PM. Association of orthodontic force system and root resorption: a systematic review. Am J Orthod Dentofacial Orthop. 2015; 147:610–626.
Article2. Weltman B, Vig KW, Fields HW, Shanker S, Kaizar EE. Root resorption associated with orthodontic tooth movement: a systematic review. Am J Orthod Dentofacial Orthop. 2010; 137:462–476.
Article3. Andreasen FM, Andreasen JO. Resorption and mineralization processes following root fracture of permanent incisors. Endod Dent Traumatol. 1988; 4:202–214.
Article4. Wedenberg C, Lindskog S. Experimental internal resorption in monkey teeth. Endod Dent Traumatol. 1985; 1:221–227.
Article5. Birkedal-Hansen H. External root resorption caused by luxation of rat molars. Scand J Dent Res. 1973; 81:47–61.
Article6. Andreasen JO. Histometric study of healing of periodontal tissues in rats after surgical injury. II. Healing events of alveolar bone, periodontal ligaments and cementum. Odontol Revy. 1976; 27:131–144.7. Casa MA, Faltin RM, Faltin K, Sander FG, Arana-Chavez VE. Root resorptions in upper first premolars after application of continuous torque moment. Intra-individual study. J Orofac Orthop. 2001; 62:285–295.
Article8. Faltin RM, Faltin K, Sander FG, Arana-Chavez VE. Ultrastructure of cementum and periodontal ligament after continuous intrusion in humans: a transmission electron microscopy study. Eur J Orthod. 2001; 23:35–49.
Article9. Choy K, Pae EK, Park Y, Kim KH, Burstone CJ. Effect of root and bone morphology on the stress distribution in the periodontal ligament. Am J Orthod Dentofacial Orthop. 2000; 117:98–105.
Article10. Killiany DM. Root resorption caused by orthodontic treatment: an evidence-based review of literature. Semin Orthod. 1999; 5:128–133.
Article11. Wesselink PR, Beertsen W, Everts V. Resorption of the mouse incisor after the application of cold to the periodontal attachment apparatus. Calcif Tissue Int. 1986; 39:11–21.
Article12. Boyde A, Ali NN, Jones SJ. Resorption of dentine by isolated osteoclasts in vitro. Br Dent J. 1984; 156:216–220.
Article13. Kameyama T, Matsumoto Y, Warita H, Otsubo K, Soma K. A mechanical stress model applied to the rat periodontium: using controlled magnitude and direction of orthodontic force with an absolute anchorage. Oral Med Pathol. 2002; 7:1–7.
Article14. Tobiume Y, Otsubo K, Soma K, Yoneyama T, Hamanaka H. Improvement in the load changeability of super-elastic Ti-Ni alloy orthodontic closed coil spring by the two-step heat treatment. J Jpn Soc Dent Mater Dev. 2000; 19:170–178.15. Kohno T, Matsumoto Y, Kanno Z, Warita H, Soma K. Experimental tooth movement under light orthodontic forces: rates of tooth movement and changes of the periodontium. J Orthod. 2002; 29:129–135.
Article16. Domon S, Shimokawa H, Matsumoto Y, Yamaguchi S, Soma K. In situ hybridization for matrix metalloproteinase-1 and cathepsin K in rat root-resorbing tissue induced by tooth movement. Arch Oral Biol. 1999; 44:907–915.
Article17. Kameyama Y, Nakane S, Maeda H, Fujita K, Takesue M, Sato E. Inhibitory effect of aspirin on root resorption induced by mechanical injury of the soft periodontal tissues in rats. J Periodontal Res. 1994; 29:113–117.
Article18. Owman-Moll P, Kurol J, Lundgren D. Continuous versus interrupted continuous orthodontic force related to early tooth movement and root resorption. Angle Orthod. 1995; 65:395–401.19. Chan E, Darendeliler MA. Physical properties of root cementum: Part 5. Volumetric analysis of root resorption craters after application of light and heavy orthodontic forces. Am J Orthod Dentofacial Orthop. 2005; 127:186–195.
Article20. Kurol J, Owman-Moll P, Lundgren D. Time-related root resorption after application of a controlled continuous orthodontic force. Am J Orthod Dentofacial Orthop. 1996; 110:303–310.
Article21. Horiuchi A, Hotokezaka H, Kobayashi K. Correlation between cortical plate proximity and apical root resorption. Am J Orthod Dentofacial Orthop. 1998; 114:311–318.
Article22. Rudolph DJ, Willes PMG, Sameshima GT. A finite element model of apical force distribution from orthodontic tooth movement. Angle Orthod. 2001; 71:127–131.23. Follin ME, Ericsson I, Thilander B. Occurrence and distribution of root resorption in orthodontically moved premolars in dogs. Angle Orthod. 1986; 56:164–175.24. Brin I, Ben-Bassat Y, Heling I, Engelberg A. The influence of orthodontic treatment on previously traumatized permanent incisors. Eur J Orthod. 1991; 13:372–377.
Article25. Parker RJ, Harris EF. Directions of orthodontic tooth movements associated with external apical root resorption of the maxillary central incisor. Am J Orthod Dentofacial Orthop. 1998; 114:677–683.
Article26. Konoo T, Kim YJ, Gu GM, King GJ. Intermittent force in orthodontic tooth movement. J Dent Res. 2001; 80:457–460.
Article27. Cooper SM, Sims MR. Evidence of acute inflammation in the periodontal ligament subsequent to orthodontic tooth movement in rats. Aust Orthod J. 1989; 11:107–109.28. Hughes B, King GJ. Effect of orthodontic appliance reactivation during the period of peak expansion in the osteoclast population. Anat Rec. 1998; 251:80–86.
Article29. Brudvik P, Rygh P. Root resorption beneath the main hyalinized zone. Eur J Orthod. 1994; 16:249–263.
Article30. Owman-Moll P, Kurol J, Lundgren D. Repair of orthodontically induced root resorption in adolescents. Angle Orthod. 1995; 65:403–408.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Photoelastic evaluation of mandibular posterior crossbite appliance
- The effet of types of orthodontic force on the root resorption and repair in rat molar
- A photoelastic study of the stress distribution in the alveolar bone by various molar uprighting springs
- Effect of orthodontic force on the amount of tooth movement and root resorption in rat
- Root resorption and bone resorption by jiggling force in cat premolars