J Gastric Cancer.
2018 Sep;18(3):264-273. 10.5230/jgc.2018.18.e29.
Who Can Perform Adjuvant Chemotherapy Treatment for Gastric Cancer? A Multicenter Retrospective Overview of the Current Status in Korea
- Affiliations
-
- 1Department of Surgery, Dongnam Institute of Radiological and Medical Sciences, Cancer Center, Busan, Korea.
- 2Department of Surgery, Korea University Medical Center, Korea University College of Medicine, Seoul, Korea. kugspss@korea.ac.kr
- 3Department of Surgery, Kyung Hee University Hospital at Gangdong, Seoul, Korea.
- 4Department of Surgery, Kosin University College of Medicine, Busan, Korea.
- 5Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- 6Department of Surgery, Dongguk University Hospital, Goyang, Korea.
- 7Department of Surgery, Soonchunhyang University Cheonan Hospital, Cheonan, Korea.
- 8Department of Surgery, Hallym University Kangdong Sacred Heart Hospital, Seoul, Korea.
- 9Department of Surgery, Chosun University College of Medicine, Gwangju, Korea.
- 10Department of Surgery, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea.
- 11Department of Surgery, Hanyang University Guri Hospital, Guri, Korea.
- 12Department of Surgery, Chung-Ang University College of Medicine, Seoul, Korea.
- 13Department of Surgery, Gyeongsang National University Hospital, Changwon, Korea.
- 14Department of Surgery, Eulji University Hospital, Daejeon, Korea.
- KMID: 2420786
- DOI: http://doi.org/10.5230/jgc.2018.18.e29
Abstract
- PURPOSE
To investigate the current status of adjuvant chemotherapy (AC) regimens in Korea and the difference in efficacy of AC administered by surgical and medical oncologists in patients with stage II or III gastric cancers.
MATERIALS AND METHODS
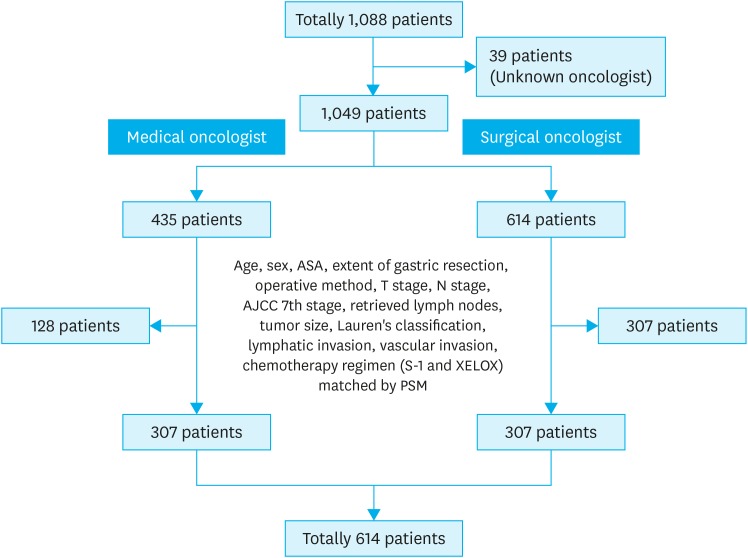
We performed a retrospective observational study among 1,049 patients who underwent curative resection and received AC for stage II and III gastric cancers between February 2012 and December 2013 at 29 tertiary referral university hospitals in Korea. To minimize the influence of potential confounders on selection bias, propensity score matching (PSM) was used based on binary logistic regression analysis. The 3-year disease-free survival (DFS) rates were compared between patients who received AC administered by medical oncologists or surgical oncologists.
RESULTS
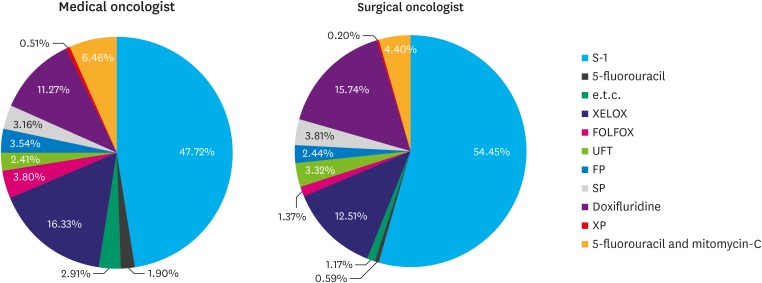
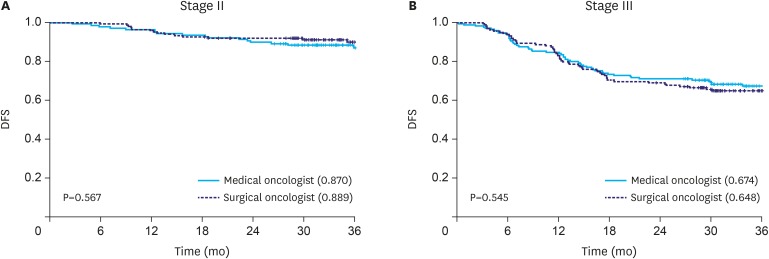
Between February 2012 and December 2013 in Korea, the most commonly prescribed AC by medical oncologists was tegafur/gimeracil/oteracil (S-1, 47.72%), followed by capecitabine with oxaliplatin (XELOX, 16.33%). After performing PSM, surgical oncologists (82.74%) completed AC as planned more often than medical oncologists (75.9%), with statistical significance (P=0.036). No difference in the 3-year DFS rates of stage II (P=0.567) or stage III (P=0.545) gastric cancer was found between the medical and surgical oncologist groups.
CONCLUSIONS
S-1 monotherapy and XELOX are a main stay of AC, regardless of whether the prescribing physician is a medical or surgical oncologist. The better compliance with AC by surgical oncologists is a valid reason to advocate that surgical oncologists perform the treatment of AC for stage II or III gastric cancers.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Treatment options for advanced gastric cancer with peritoneal metastasis: experience from a single institution in Korea
Dong-Wook Kim, Sang Il Youn, Ye Seob Jee
Ann Surg Treat Res. 2021;100(4):209-217. doi: 10.4174/astr.2021.100.4.209.
Reference
-
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386. PMID: 25220842.
Article2. Gallo A, Cha C. Updates on esophageal and gastric cancers. World J Gastroenterol. 2006; 12:3237–3242. PMID: 16718845.
Article3. Gunderson LL. Gastric cancer--patterns of relapse after surgical resection. Semin Radiat Oncol. 2002; 12:150–161. PMID: 11979416.
Article4. GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group. Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010; 303:1729–1737. PMID: 20442389.5. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–1820. PMID: 17978289.
Article6. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011; 29:4387–4393. PMID: 22010012.
Article7. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012; 379:315–321. PMID: 22226517.
Article8. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474. PMID: 20180029.
Article9. Cho JH, Lim JY, Cho JY. Comparison of capecitabine and oxaliplatin with S-1 as adjuvant chemotherapy in stage III gastric cancer after D2 gastrectomy. PLoS One. 2017; 12:e0186362. PMID: 29040299.
Article10. Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016; 17:309–318. PMID: 26822397.
Article11. Aoyama T, Kawabe T, Fujikawa H, Hayashi T, Yamada T, Tsuchida K, et al. Loss of lean body mass as an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol. 2015; 22:2560–2566. PMID: 25515199.
Article12. Yamashita K, Kurokawa Y, Yamamoto K, Hirota M, Kawabata R, Mikami J, et al. Risk factors for poor compliance with adjuvant S-1 chemotherapy for gastric cancer: a multicenter retrospective study. Ann Surg Oncol. 2017; 24:2639–2645. PMID: 28608116.
Article13. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017; 20:1–19.14. Malet-Martino M, Martino R. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): a review. Oncologist. 2002; 7:288–323. PMID: 12185293.
Article15. Davis JL, Ripley RT. Postgastrectomy syndromes and nutritional considerations following gastric surgery. Surg Clin North Am. 2017; 97:277–293. PMID: 28325187.
Article16. Iacovelli R, Pietrantonio F, Palazzo A, Maggi C, Ricchini F, de Braud F, et al. Incidence and relative risk of grade 3 and 4 diarrhoea in patients treated with capecitabine or 5-fluorouracil: a meta-analysis of published trials. Br J Clin Pharmacol. 2014; 78:1228–1237. PMID: 24962653.
Article17. Berg P, McCallum R. Dumping syndrome: a review of the current concepts of pathophysiology, diagnosis, and treatment. Dig Dis Sci. 2016; 61:11–18. PMID: 26396002.
Article18. Ahmed H, Hammad AM, Abushouk AI, Zidan M, Salem M, Negida A, et al. Meta-analysis of safety and efficacy of rolapitant, NK-1 receptor antagonist for prevention of chemotherapy-induced nausea and vomiting. Curr Probl Cancer. 2018; 42:241–255. PMID: 29310827.
Article19. Hayashi S, Fujii M, Takayama T. Prevention of postoperative small bowel obstruction in gastric cancer. Surg Today. 2015; 45:1352–1359. PMID: 25542082.
Article