Obstet Gynecol Sci.
2018 Mar;61(2):267-273. 10.5468/ogs.2018.61.2.267.
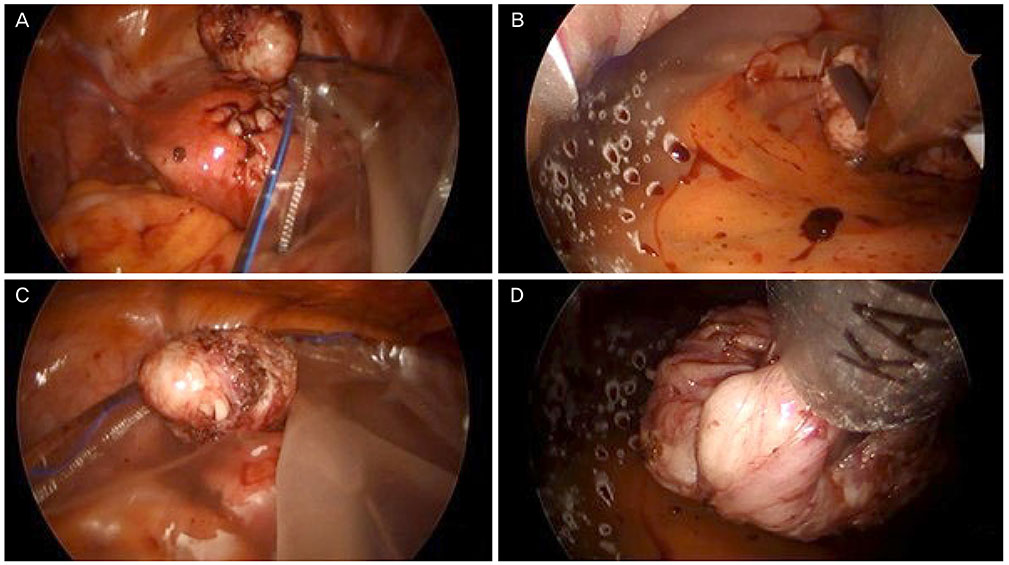
In-bag power morcellation technique in single-port laparoscopic myomectomy
- Affiliations
-
- 1Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Women's Cancer Center, Yonsei Cancer Center, Institute of Women's Life Medical Science, Yonsei University College of Medicine, Seoul, Korea. SAN1@yuhs.ac
- KMID: 2420182
- DOI: http://doi.org/10.5468/ogs.2018.61.2.267
Abstract
OBJECTIVE
This study introduces and evaluates the feasibility, safety, and surgical outcomes of the in-bag power morcellation technique during single-port assisted (SPA) laparoscopic myomectomy in comparison with manual scalpel morcellation.
METHODS
This is a retrospective review of a total of 58 patients who underwent SPA laparoscopic myomectomy employing in-bag power morcellation (n=27) or manual scalpel morcellation (n=31), performed between December 2014 and December 2016. Surgical outcomes, including total operation time, estimated blood loss, postoperative hemoglobin changes, postoperative hospital stay, postoperative pain (visual analog scale), perioperative and postoperative complications were evaluated.
RESULTS
The demographics and patient characteristics were similar between both groups. The median patient age was 34 years and median body mass index was 20.84 kg/m2. The median specimen weight was 110 g. The median operating time was 138 minutes. The median estimated blood loss was 50 mL and the median postoperative hemoglobin change was 2.2 g/dL. The median postoperative hospital stay was 2 days and the median postoperative pain scores were 5 after 6 hours, 3 after 24 hours, and 2 after 48 hours. Occult malignancy was not identified in any patients. There were no intraoperative complications such as LapBag ruptures or gross spillage.
CONCLUSION
In-bag power morcellation for SPA laparoscopic myomectomy is feasible and safe, minimizing the risks of open power morcellation. There were also no statistically significant differences in surgical outcomes.
MeSH Terms
Figure
Reference
-
1. Bhave Chittawar P, Franik S, Pouwer AW, Farquhar C. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2014; CD004638.
Article2. Nieboer TE, Johnson N, Lethaby A, Tavender E, Curr E, Garry R, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009; CD003677.
Article3. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012; 16:519–524.
Article4. Parker WH. Indications for morcellation in gynecologic surgery. Curr Opin Obstet Gynecol. 2018; 30:75–80.
Article5. Steiner RA, Wight E, Tadir Y, Haller U. Electrical cutting device for laparoscopic removal of tissue from the abdominal cavity. Obstet Gynecol. 1993; 81:471–474.6. Carter JE, McCarus SD. Laparoscopic myomectomy. Time and cost analysis of power vs. manual morcellation. J Reprod Med. 1997; 42:383–388.7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014; 21:486–491.
Article8. Kho KA, Nezhat CH. Evaluating the risks of electric uterine morcellation. JAMA. 2014; 311:905–906.
Article9. Graebe K, Garcia-Soto A, Aziz M, Valarezo V, Heller PB, Tchabo N, et al. Incidental power morcellation of malignancy: a retrospective cohort study. Gynecol Oncol. 2015; 136:274–277.
Article10. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication [Internet]. Silver Spring (MD): US Food and Drug Administration;2014. cited 2017 Mar 27. Available from: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm.11. Yim GW, Jung YW, Paek J, Lee SH, Kwon HY, Nam EJ, et al. Transumbilical single-port access versus conventional total laparoscopic hysterectomy: surgical outcomes. Am J Obstet Gynecol. 2010; 203:26.e1–26.e6.
Article12. Kho KA, Anderson TL, Nezhat CH. Intracorporeal electromechanical tissue morcellation: a critical review and recommendations for clinical practice. Obstet Gynecol. 2014; 124:787–793.13. Leren V, Langebrekke A, Qvigstad E. Parasitic leiomyomas after laparoscopic surgery with morcellation. Acta Obstet Gynecol Scand. 2012; 91:1233–1236.
Article14. Anupama R, Ahmad SZ, Kuriakose S, Vijaykumar DK, Pavithran K, Seethalekshmy NV. Disseminated peritoneal leiomyosarcomas after laparoscopic “myomectomy” and morcellation. J Minim Invasive Gynecol. 2011; 18:386–389.
Article15. AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL practice report: morcellation during uterine tissue extraction. J Minim Invasive Gynecol. 2014; 21:517–530.16. Nezhat F, Apostol R, Greene AD, Pilkinton ML. To morcellate or not to morcellate: a cross-sectional survey of gynecologic surgeons. JSLS. 2017; 21:e2016.00092.
Article17. Cohen SL, Einarsson JI, Wang KC, Brown D, Boruta D, Scheib SA, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014; 124:491–497.
Article18. Serur E, Zambrano N, Brown K, Clemetson E, Lakhi N. Extracorporeal manual morcellation of very large uteri within an enclosed endoscopic bag: our 5-year experience. J Minim Invasive Gynecol. 2016; 23:903–908.19. Srouji SS, Kaser DJ, Gargiulo AR. Techniques for contained morcellation in gynecologic surgery. Fertil Steril. 2015; 103:e34.
Article20. Vargas MV, Cohen SL, Fuchs-Weizman N, Wang KC, Manoucheri E, Vitonis AF, et al. Open power morcellation versus contained power morcellation within an insufflated isolation bag: comparison of perioperative outcomes. J Minim Invasive Gynecol. 2015; 22:433–438.
Article21. American College of Obstetricians and Gynecologists. Executive summary. Power morcellation and occult malignancy in gynecologic surgery: a special report. Silver Spring (MD): US Food and Drug Administration;2014.22. American Association of Gynecologic Laparoscopists. AAGL statement to the FDA on power morcellation. Cypress (CA): American Association of Gynecologic Laparoscopists;2014.23. US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. Silver Spring (MD): US Food and Drug Administration;2016.