Allergy Asthma Immunol Res.
2018 Sep;10(5):478-489. 10.4168/aair.2018.10.5.478.
Measurement of Exhaled Nitric Oxide in Children: A Comparison Between NObreath® and NIOX VERO® Analyzers
- Affiliations
-
- 1Department of Allergy, Clinical Research Center for Allergy and Rheumatology, Sagamihara National Hospital, Kanagawa, Japan.
- 2Department of Pediatrics, St. Marianna University School of Medicine, Kanagawa, Japan.
- 3Department of Pediatrics, Sagamihara National Hospital, Kanagawa, Japan. n-yanagida@sagamihara-hosp.gr.jp
- 4Course of Allergy and Clinical Immunology, Juntendo University Graduate School of Medicine, Tokyo, Japan.
- KMID: 2418046
- DOI: http://doi.org/10.4168/aair.2018.10.5.478
Abstract
- PURPOSE
Few studies have compared fractional exhaled nitric oxide (FeNO) measurement by NIOX VERO® (NOV) and other devices in children. Moreover, there is no agreement between differences in FeNO values obtained using different devices in adults. Here, we compared FeNO values obtained using NOV and NObreath® (NOB) systems to derive a correction equation for children.
METHODS
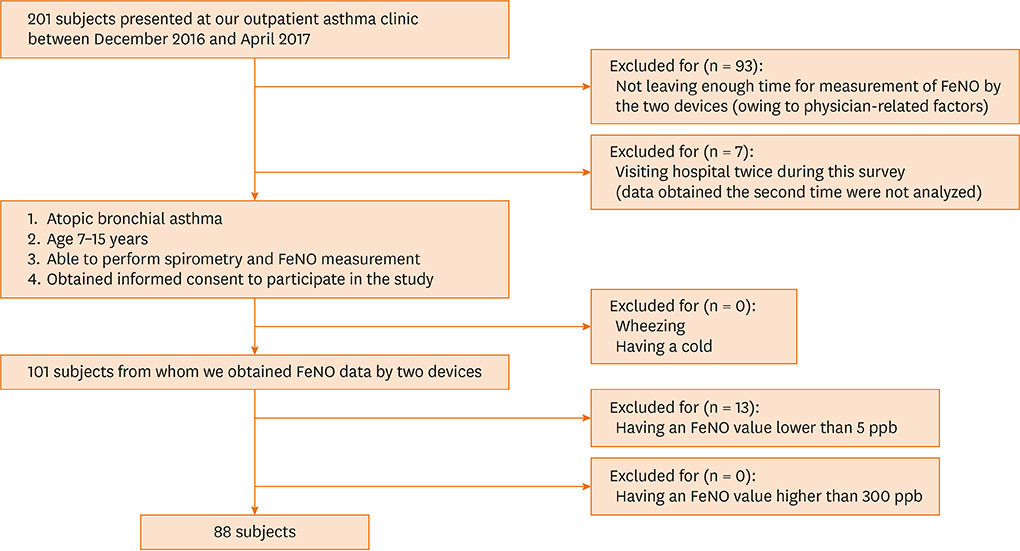
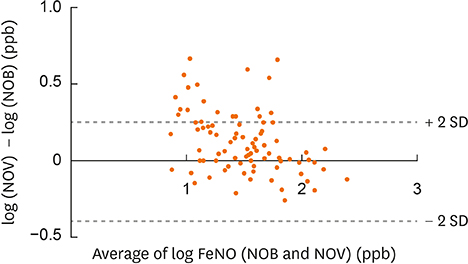
Eighty-eight participants (age 7-15 years) who were diagnosed with atopic bronchial asthma and visited Sagamihara National Hospital as outpatients between January and April of 2017 were included. We measured FeNO values obtained using NOB and NOV, and analyzed them using Wilcoxon tests and Altman-Bland plots.
RESULTS
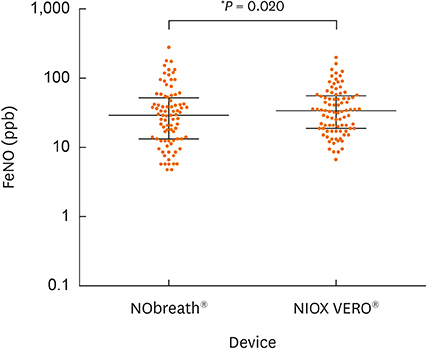
The median age of the participants was 11.5 years, and the scored Asthma Control Test (ACT) or Childhood ACT (C-ACT) was 25 (interquartile range, 24-25) or 26 (24-27). NOB and NOV values were significantly different (31 [14-52] versus 36 [20-59] ppb; P = 0.020) and strongly correlated (r = 0.92). An equation to convert NOB values into NOV values was derived using linear regression as follows: log NOV = 0.7329 × log NOB + 0.4704; NOB for 20, 40, 58, 80 and 100 ppb corresponded to NOV for 27, 44, 59, 73 and 86 ppb. Thus, NOB < 58 ppb suggested NOB < NOV, whereas NOB > 58 ppb suggested NOB > NOV.
CONCLUSIONS
NOB and NOV values were strongly correlated. Participants whose FeNO values were relatively low represented NOB < NOV, whereas those whose FeNO values were relatively high represented NOB > NOV.
Keyword
Figure
Cited by 1 articles
-
Short-term Haze Exposure Predisposes Healthy Volunteers to Nasal Inflammation
Mu Xian, Kuiji Wang, Hongfei Lou, Yang Wang, Luo Zhang, Chengshuo Wang
Allergy Asthma Immunol Res. 2019;11(5):632-643. doi: 10.4168/aair.2019.11.5.632.
Reference
-
1. Pohunek P, Warner JO, Turzíková J, Kudrmann J, Roche WR. Markers of eosinophilic inflammation and tissue re-modelling in children before clinically diagnosed bronchial asthma. Pediatr Allergy Immunol. 2005; 16:43–51.
Article2. Smith AD, Cowan JO, Filsell S, McLachlan C, Monti-Sheehan G, Jackson P, et al. Diagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional tests. Am J Respir Crit Care Med. 2004; 169:473–478.3. Smith AD, Cowan JO, Brassett KP, Filsell S, McLachlan C, Monti-Sheehan G, et al. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med. 2005; 172:453–459.4. Knuffman JE, Sorkness CA, Lemanske RF Jr, Mauger DT, Boehmer SJ, Martinez FD, et al. Phenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthma. J Allergy Clin Immunol. 2009; 123:411–416.
Article5. Pijnenburg MW, Hofhuis W, Hop WC, De Jongste JC. Exhaled nitric oxide predicts asthma relapse in children with clinical asthma remission. Thorax. 2005; 60:215–218.
Article6. American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005; 171:912–930.7. Kim HB, Eckel SP, Kim JH, Gilliland FD. Exhaled NO: determinants and clinical application in children with allergic airway disease. Allergy Asthma Immunol Res. 2016; 8:12–21.
Article8. Yoon J, Choi YJ, Lee E, Cho HJ, Yang SI, Kim YH, et al. Allergic rhinitis in preschool children and the clinical utility of FeNO. Allergy Asthma Immunol Res. 2017; 9:314–321.
Article9. Pisi R, Aiello M, Tzani P, Marangio E, Olivieri D, Chetta A. Measurement of fractional exhaled nitric oxide by a new portable device: comparison with the standard technique. J Asthma. 2010; 47:805–809.
Article10. Kapande KM, McConaghy LA, Douglas I, McKenna S, Hughes JL, McCance DR, et al. Comparative repeatability of two handheld fractional exhaled nitric oxide monitors. Pediatr Pulmonol. 2012; 47:546–550.
Article11. Fukuhara A, Saito J, Sato S, Sato Y, Nikaido T, Saito K, et al. Validation study of asthma screening criteria based on subjective symptoms and fractional exhaled nitric oxide. Ann Allergy Asthma Immunol. 2011; 107:480–486.
Article12. Tsuburai T, Kamide Y, Nakamura Y, Tomita Y, Hamada Y, Watai K, et al. Differences in fraction of exhaled nitric oxide values measured by two hand-held analyzers (NO breath® and NIOX Vero®). Arerugi. 2017; 66:204–208.13. National Institute for Health and Care Excellence. NICE Guidance [Internet]. London: National Institute for Health and Care Excellence;2017. cited 2017 Aug 16. Available from: https://www.nice.org.uk/Guidance.14. Buchvald F, Baraldi E, Carraro S, Gaston B, De Jongste J, Pijnenburg MW, et al. Measurements of exhaled nitric oxide in healthy subjects age 4 to 17 years. J Allergy Clin Immunol. 2005; 115:1130–1136.
Article15. Hamasaki Y, Kohno Y, Ebisawa M, Kondo N, Nishima S, Nishimuta T, et al. Japanese pediatric guideline for the treatment and management of bronchial asthma 2012. Pediatr Int. 2014; 56:441–450.
Article16. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention: NHLBI_WHO workshop report. National Heart, Lung, and Blood Institute, National Institutes of Health [Internet]. place unknown: Global Initiative for Asthma;2009. cited 2009. Available from: http://www.ginasthma.org.17. Expert Panel Report 3 (EPR3): Guidelines for the Diagnosis and Management of Asthma. National Heart, Lung and Blood Institute, National Institutes of Health;2007. NIH Publication Number 08-5846.18. Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004; 113:59–65.19. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1:307–310.
Article20. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011; 184:602–615.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Exhaled Nitric Oxide Analyzers in Childhood Asthma
- Measurement of Fractional Exhaled Nitric Oxide in Adults: Comparison of Two Different Analyzers (NIOX VERO and NObreath)
- Fractional Exhaled Nitric Oxide: Comparison Between Portable Devices and Correlation With Sputum Eosinophils
- Exhaled Nitric Oxide Measurement for Asthma Management
- Measurement and Interpretation of Fractional Exhaled Nitric Oxide