Cancer Res Treat.
2018 Jul;50(3):956-963. 10.4143/crt.2017.181.
Comparison of Clinical Features and Outcomes in Epithelial Ovarian Cancer according to Tumorigenicity in Patient-Derived Xenograft Models
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Institute of Women's Life Medical Science, Yonsei University College of Medicine, Seoul, Korea. ytkchoi@yuhs.ac, NAHMEJ6@yuhs.ac
- 2Department of Obstetrics and Gynecology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2417884
- DOI: http://doi.org/10.4143/crt.2017.181
Abstract
- PURPOSE
Although the use of xenograft models is increasing, few studies have compared the clinical features or outcomes of epithelial ovarian cancer (EOC) patients according to the tumorigenicity of engrafted specimens. The purpose of this study was to evaluate whether tumorigenicity was associated with the clinical features and outcomes of EOC patients.
MATERIALS AND METHODS
Eighty-eight EOC patients who underwent primary or interval debulking surgery from June 2014 to December 2015 were included. Fresh tumor specimens were implanted subcutaneously on each flank of immunodeficient mice. Patient characteristics, progression-free survival (PFS), and germline mutation spectra were compared according to tumorigenicity.
RESULTS
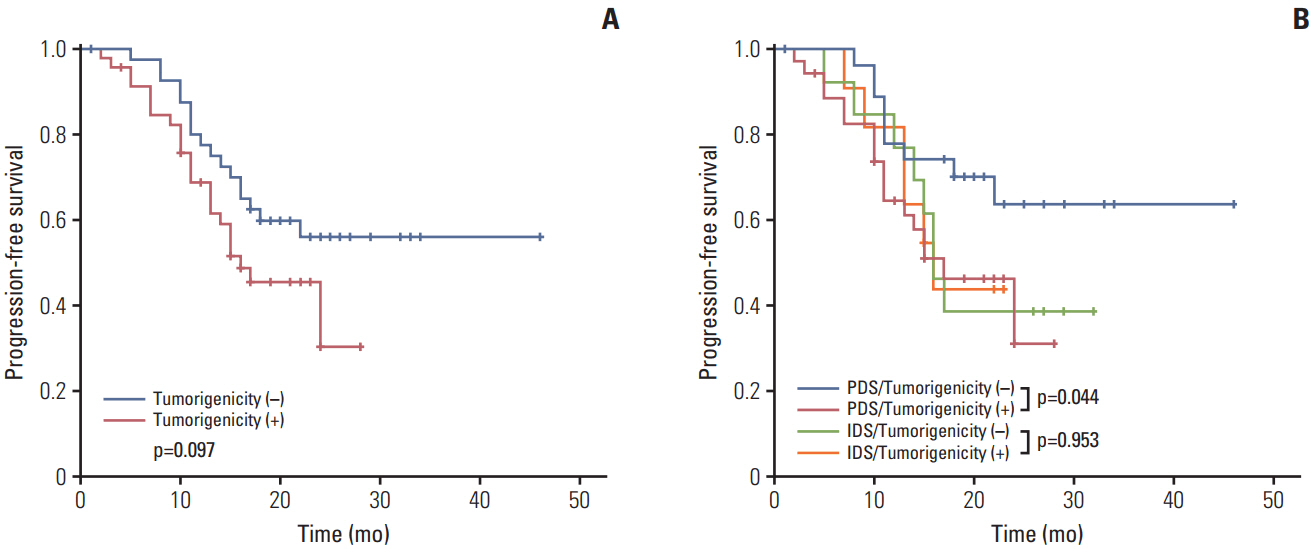
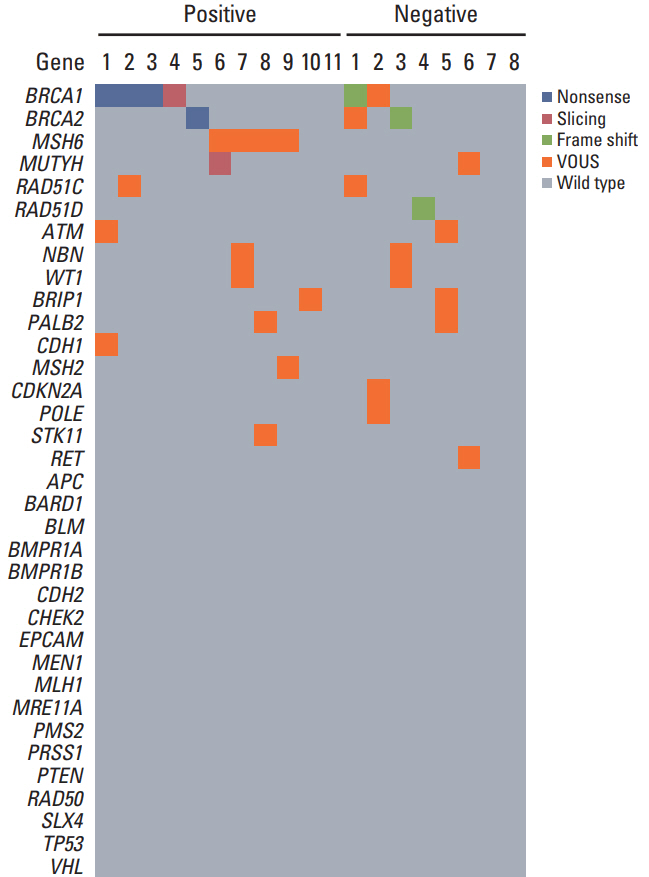
Xenografts were established successfully from 49 of 88 specimens. Tumorigenicity was associated with lymphovascular invasion and there was a propensity to engraft successfully with high-grade tumors. Tumors from patientswho underwent non-optimal (residual disease ≥ 1 cm) primary orinterval debulking surgery had a significantly greater propensity to achieve tumorigenicity than those who received optimal surgery. In addition, patients whose tumors became engrafted seemed to have a shorter PFS and more frequent germline mutations than patients whose tumors failed to engraft. Tumorigenicity was a significant factor for predicting PFS with advanced International Federation of Gynecology and Obstetrics stage and high-grade cancers.
CONCLUSION
sTumorigenicity in a xenograft model was a strong prognostic factor and was associated with more aggressive tumors in EOC patients. Xenograft models can be useful as a preclinical tool to predict prognosis and could be applied to further pharmacologic and genomic studies on personalized treatments.
MeSH Terms
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30.
Article2. Erriquez J, Olivero M, Mittica G, Scalzo MS, Vaira M, De Simone M, et al. Xenopatients show the need for precision medicine approach to chemotherapy in ovarian cancer. Oncotarget. 2016; 7:26181–91.
Article3. Nemati F, Sastre-Garau X, Laurent C, Couturier J, Mariani P, Desjardins L, et al. Establishment and characterization of a panel of human uveal melanoma xenografts derived from primary and/or metastatic tumors. Clin Cancer Res. 2010; 16:2352–62.
Article4. Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc. 2009; 4:1670–80.
Article5. Rubio-Viqueira B, Hidalgo M. Direct in vivo xenograft tumor model for predicting chemotherapeutic drug response in cancer patients. Clin Pharmacol Ther. 2009; 85:217–21.
Article6. Marangoni E, Vincent-Salomon A, Auger N, Degeorges A, Assayag F, de Cremoux P, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res. 2007; 13:3989–98.
Article7. Puig I, Chicote I, Tenbaum SP, Arques O, Herance JR, Gispert JD, et al. A personalized preclinical model to evaluate the metastatic potential of patient-derived colon cancer initiating cells. Clin Cancer Res. 2013; 19:6787–801.
Article8. Dobbin ZC, Katre AA, Steg AD, Erickson BK, Shah MM, Alvarez RD, et al. Using heterogeneity of the patient-derived xenograft model to identify the chemoresistant population in ovarian cancer. Oncotarget. 2014; 5:8750–64.
Article9. Weroha SJ, Becker MA, Enderica-Gonzalez S, Harrington SC, Oberg AL, Maurer MJ, et al. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin Cancer Res. 2014; 20:1288–97.
Article10. Heo EJ, Cho YJ, Cho WC, Hong JE, Jeon HK, Oh DY, et al. Patient-derived xenograft models of epithelial ovarian cancer for preclinical studies. Cancer Res Treat. 2017; 49:915–26.
Article11. Oh BY, Lee WY, Jung S, Hong HK, Nam DH, Park YA, et al. Correlation between tumor engraftment in patient-derived xenograft models and clinical outcomes in colorectal cancer patients. Oncotarget. 2015; 6:16059–68.
Article12. Medina-Franco H, Cortes-Gonzalez R, Lambreton-Hinojosa F, Fimbres-Morales A, Vargas-Siordia JC. Neoadjuvant chemotherapy increases R0 cytoreduction rate but does not improve final outcome in advanced epithelial ovarian cancer. Ann Surg Oncol. 2017; 24:1330–5.
Article13. Ataseven B, Grimm C, Harter P, Heitz F, Traut A, Prader S, et al. Prognostic impact of debulking surgery and residual tumor in patients with epithelial ovarian cancer FIGO stage IV. Gynecol Oncol. 2016; 140:215–20.
Article14. John T, Kohler D, Pintilie M, Yanagawa N, Pham NA, Li M, et al. The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early-stage non-small cell lung cancer. Clin Cancer Res. 2011; 17:134–41.
Article15. Anderson TM, Hess SD, Egilmez NK, Nwogu CE, Lenox JM, Bankert RB. Comparison of human lung cancer/SCID mouse tumor xenografts and cell culture growth with patient clinical outcomes. J Cancer Res Clin Oncol. 2003; 129:565–8.
Article16. DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011; 17:1514–20.
Article17. Bobbs AS, Cole JM, Cowden Dahl KD. Emerging and evolving ovarian cancer animal models. Cancer Growth Metastasis. 2015; 8(Suppl 1):29–36.
Article18. Topp MD, Hartley L, Cook M, Heong V, Boehm E, McShane L, et al. Molecular correlates of platinum response in human high-grade serous ovarian cancer patient-derived xenografts. Mol Oncol. 2014; 8:656–68.
Article19. Ricci F, Bizzaro F, Cesca M, Guffanti F, Ganzinelli M, Decio A, et al. Patient-derived ovarian tumor xenografts recapitulate human clinicopathology and genetic alterations. Cancer Res. 2014; 74:6980–90.
Article20. Li H, Zhu Y, Tang X, Li J, Li Y, Zhong Z, et al. Integrated analysis of transcriptome in cancer patient-derived xenografts. PLoS One. 2015; 10:e0124780.
Article21. Malaney P, Nicosia SV, Dave V. One mouse, one patient paradigm: New avatars of personalized cancer therapy. Cancer Lett. 2014; 344:1–12.
Article22. Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012; 9:338–50.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sex-dependent liver cancer xenograft models for predicting clinical data in the evaluation of anticancer drugs
- Canine as a Comparative and Translational Model for Human Mammary Tumor
- Anti-cancer effect of fenbendazole-incorporated PLGA nanoparticles in ovarian cancer
- Validity of patient-derived xenograft mouse models for lung cancer based on exome sequencing data
- Comparison of the Genetic Alterations between Primary Colorectal Cancers and Their Corresponding Patient-Derived Xenograft Tissues