Cancer Res Treat.
2018 Jul;50(3):701-711. 10.4143/crt.2017.180.
Pretreatment Serum Amyloid A and C-reactive Protein Comparing with Epstein-Barr Virus DNA as Prognostic Indicators in Patients with Nasopharyngeal Carcinoma: A Prospective Study
- Affiliations
-
- 1Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China. maihq@sysucc.org.cn
- 2Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, China.
- 3Department of Oncology, the First Affiliated Hospital, Jinan University, Guangzhou, China.
- 4Department of Information Technology, Sun Yat-Sen University Cancer Center, Guangzhou, China.
- KMID: 2417859
- DOI: http://doi.org/10.4143/crt.2017.180
Abstract
- PURPOSE
The measuring Epstein-Barr virus (EBV) DNA is an important predictor of nasopharyngeal carcinoma (NPC). This study evaluated the predictive value of pretreatment serum amyloid A (SAA) and C-reactive protein (CRP) comparing with EBV DNA in patients with NPC.
MATERIALS AND METHODS
In an observational study of 419 non-metastatic NPC patients, we prospectively evaluated the prognostic effects of pretreatment SAA, CRP, and EBV DNA on survival. The primary end-point was progress-free survival (PFS).
RESULTS
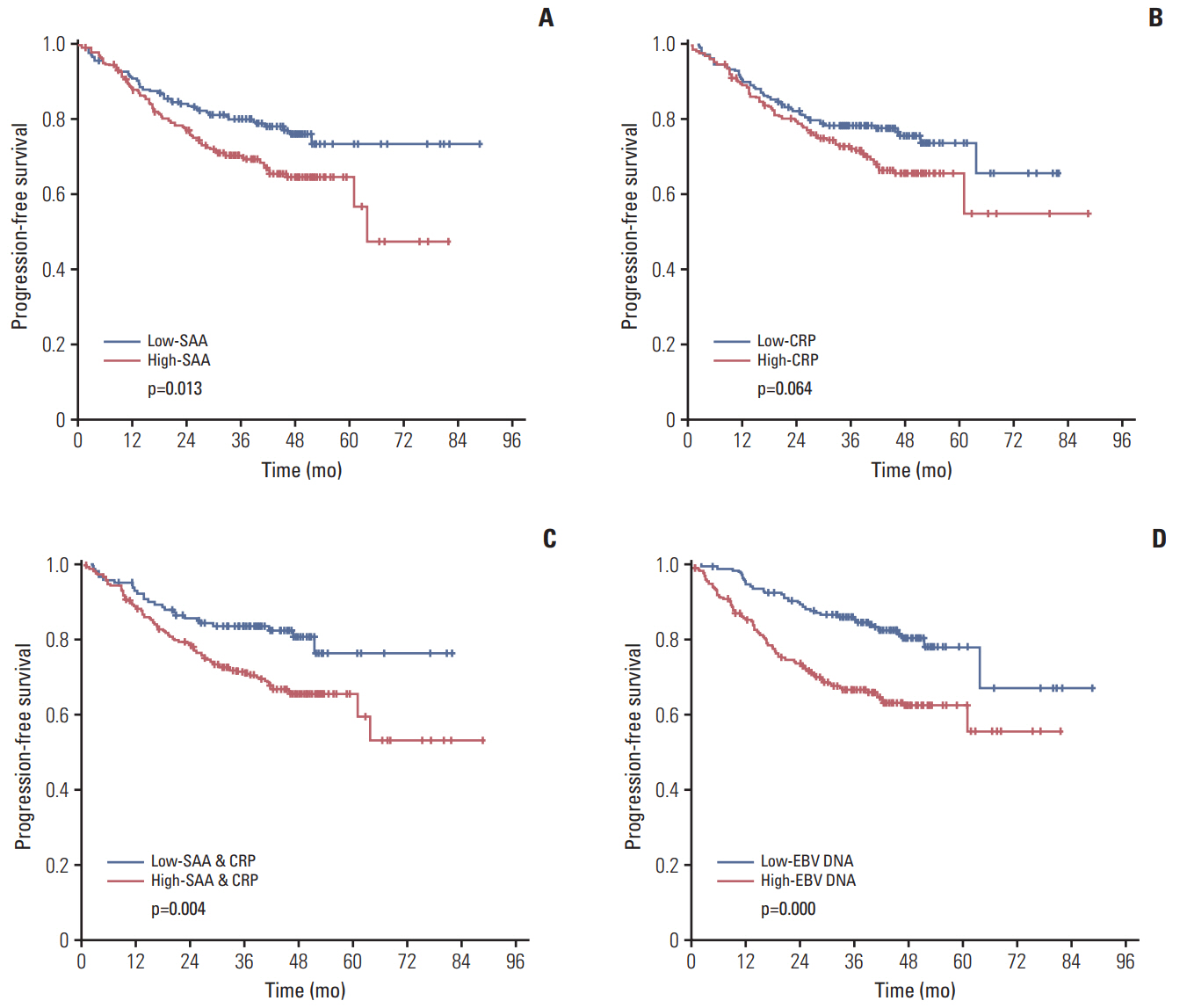
The median level of SAA and CRP was 4.28 mg/L and 1.88 mg/L, respectively. For the high-SAA group (> 4.28 mg/L) versus the low-SAA (≤ 4.28 mg/L) group and the high-CRP group (> 1.88 mg/L) versus the low-CRP (≤ 1.88 mg/L) group, the 5-year PFS was 64.5% versus 73.1% (p=0.013) and 65.2% versus 73.3% (p=0.064), respectively. EBV DNA detection showed a superior predictive result, the 5-year PFS in the EBV DNA ≥ 1,500 copies/mL group was obviously different than the EBV DNA < 1,500 copies/mL group (62.2% versus 77.8%, p < 0.001). Multifactorial Cox regression analysis confirmed that in the PFS, the independent prognostic factors were including EBV DNA (hazard ratio [HR], 1.788; p=0.009), tumour stage (HR, 1.903; p=0.021), and node stage (HR, 1.498; p=0.049), but the SAA and CRP were not included in the independent prognostic factors.
CONCLUSION
The results of SAA and CRP had a certain relationship with the prognosis of NPC, and the prognosis of patients with high level of SAA and CRP were poor. However, the predictive ability of SAA and CRP was lower than that of EBV DNA.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Wee JT, Ha TC, Loong SL, Qian CN. Is nasopharyngeal cancer really a "Cantonese cancer"? Chin J Cancer. 2010; 29:517–26.
Article2. Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015; 16:645–55.
Article3. Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005; 365:2041–54.
Article4. Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005; 5:845–56.
Article5. Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004; 350:2461–70.
Article6. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002; 420:860–7.
Article7. Schultz DR, Arnold PI. Properties of four acute phase proteins: C-reactive protein, serum amyloid A protein, alpha 1-acid glycoprotein, and fibrinogen. Semin Arthritis Rheum. 1990; 20:129–47.8. Chan DC, Chen CJ, Chu HC, Chang WK, Yu JC, Chen YJ, et al. Evaluation of serum amyloid A as a biomarker for gastric cancer. Ann Surg Oncol. 2007; 14:84–93.
Article9. Cho WC, Yip TT, Yip C, Yip V, Thulasiraman V, Ngan RK, et al. Identification of serum amyloid a protein as a potentially useful biomarker to monitor relapse of nasopharyngeal cancer by serum proteomic profiling. Clin Cancer Res. 2004; 10(1 Pt 1):43–52.
Article10. Howard BA, Wang MZ, Campa MJ, Corro C, Fitzgerald MC, Patz EF Jr. Identification and validation of a potential lung cancer serum biomarker detected by matrix-assisted laser desorption/ ionization-time of flight spectra analysis. Proteomics. 2003; 3:1720–4.11. Liao Q, Zhao L, Chen X, Deng Y, Ding Y. Serum proteome analysis for profiling protein markers associated with carcinogenesis and lymph node metastasis in nasopharyngeal carcinoma. Clin Exp Metastasis. 2008; 25:465–76.
Article12. Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009; 27:3437–44.
Article13. Tang LQ, Li CF, Chen QY, Zhang L, Lai XP, He Y, et al. High-sensitivity C-reactive protein complements plasma EpsteinBarr virus deoxyribonucleic acid prognostication in nasopharyngeal carcinoma: a large-scale retrospective and prospective cohort study. J Clin Oncol. 2009; 27:3437–44.
Article14. Xia WX, Ye YF, Lu X, Wang L, Ke LR, Zhang HB, et al. The impact of baseline serum C-reactive protein and C-reactive protein kinetics on the prognosis of metastatic nasopharyngeal carcinoma patients treated with palliative chemotherapy. PLoS One. 2013; 8:e76958.
Article15. An X, Wang FH, Ding PR, Deng L, Jiang WQ, Zhang L, et al. Plasma Epstein-Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer. 2011; 117:3750–7.
Article16. Yamada T. Serum amyloid A (SAA): a concise review of biology, assay methods and clinical usefulness. Clin Chem Lab Med. 1999; 37:381–8.
Article17. Malle E, De Beer FC. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. Eur J Clin Invest. 1996; 26:427–35.
Article18. Biran H, Friedman N, Neumann L, Pras M, Shainkin-Kestenbaum R. Serum amyloid A (SAA) variations in patients with cancer: correlation with disease activity, stage, primary site, and prognosis. J Clin Pathol. 1986; 39:794–7.
Article19. Liu DH, Wang XM, Zhang LJ, Dai SW, Liu LY, Liu JF, et al. Serum amyloid A protein: a potential biomarker correlated with clinical stage of lung cancer. Biomed Environ Sci. 2007; 20:33–40.20. Wood SL, Rogers M, Cairns DA, Paul A, Thompson D, Vasudev NS, et al. Association of serum amyloid A protein and peptide fragments with prognosis in renal cancer. Br J Cancer. 2010; 103:101–11.
Article21. Vermaat JS, Gerritse FL, van der Veldt AA, Roessingh WM, Niers TM, Oosting SF, et al. Validation of serum amyloid alpha as an independent biomarker for progression-free and overall survival in metastatic renal cell cancer patients. Eur Urol. 2012; 62:685–95.22. Cho WC, Yip TT, Cheng WW, Au JS. Serum amyloid A is elevated in the serum of lung cancer patients with poor prognosis. Br J Cancer. 2010; 102:1731–5.
Article23. Sung HJ, Ahn JM, Yoon YH, Rhim TY, Park CS, Park JY, et al. Identification and validation of SAA as a potential lung cancer biomarker and its involvement in metastatic pathogenesis of lung cancer. J Proteome Res. 2011; 10:1383–95.
Article24. Toriola AT, Cheng TY, Neuhouser ML, Wener MH, Zheng Y, Brown E, et al. Biomarkers of inflammation are associated with colorectal cancer risk in women but are not suitable as early detection markers. Int J Cancer. 2013; 132:2648–58.
Article25. Cocco E, Bellone S, El-Sahwi K, Cargnelutti M, Casagrande F, Buza N, et al. Serum amyloid A (SAA): a novel biomarker for uterine serous papillary cancer. Br J Cancer. 2009; 101:335–41.
Article26. Leung SF, Zee B, Ma BB, Hui EP, Mo F, Lai M, et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. 2006; 24:5414–8.
Article27. Findeisen P, Zapatka M, Peccerella T, Matzk H, Neumaier M, Schadendorf D, et al. Serum amyloid A as a prognostic marker in melanoma identified by proteomic profiling. J Clin Oncol. 2009; 27:2199–208.
Article28. Pierce BL, Neuhouser ML, Wener MH, Bernstein L, Baumgartner RN, Ballard-Barbash R, et al. Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Breast Cancer Res Treat. 2009; 114:155–67.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Value of Serum Epstein-Barr Virus Antibodies and Their Correlation with TNM Classification in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma
- Gene Cloning of the Epstein-Barr Virus (EBV) Antigen Reactive with the Serum from EBV-infected Patients
- Detection of Epstein-Barr Virus DNA in Oropharynx by Polymerase Chain Reaction
- Detection of Epstein-Barr virus DNA in nasopharyngeal cancer by polymerase chain reaction
- Correlation between Expression of p53 and Bcl-2 Protein and Epstein-Barr Virus Detection in Gastric Adenocarcinoma