Intest Res.
2018 Apr;16(2):273-281. 10.5217/ir.2018.16.2.273.
Evaluation of the drug-induced lymphocyte stimulation test for diagnosing mesalazine allergy
- Affiliations
-
- 1The Third Department of Internal Medicine, Kyorin University School of Medicine, Mitaka, Japan. thisamatsu@ks.kyorin-u.ac.jp
- KMID: 2417679
- DOI: http://doi.org/10.5217/ir.2018.16.2.273
Abstract
- BACKGROUND/AIMS
Mesalazine is an effective drug for treating ulcerative colitis (UC), but causes allergic symptoms in a few cases. Therefore, the objective of this study was to evaluate the usefulness of the drug-induced lymphocyte stimulation test (DLST) for the diagnosis of mesalazine allergy.
METHODS
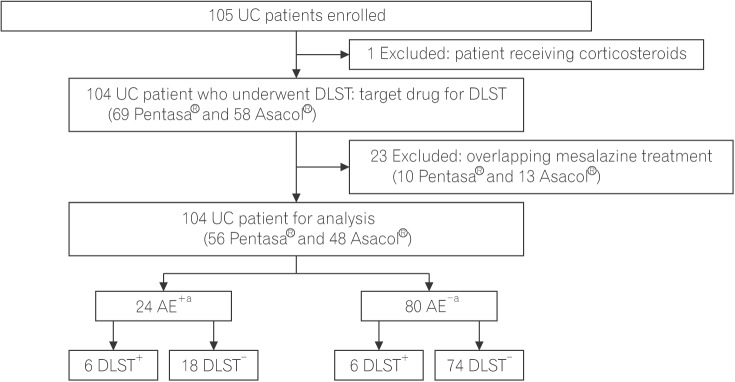
Patients with UC treated with mesalazine with or without a history of associated adverse events (AEs) were enrolled at Kyorin University Hospital from July 2016 to April 2017.
RESULTS
The DLST was performed in 104 patients with UC, of which 24 had a history of AEs due to mesalazine treatment. The control value of DLST was 337.4±296.3 counts per minute (cpm) in the AE+ group and 408.0±371.9 cpm in the AE− group. The measured value of DLST was 578.8±424.7 cpm in the AE+ group and 476.5±471.8 cpm in the AE− group. The stimulation index (SI) was 243.9%±291.1% in the AE+ group and 119.8%±53.0% in the AE− group. The SI value and DLST positivity were significantly higher in the AE+ group than in the AE− group (P=0.030 and P=0.029, respectively). The test sensitivity and specificity were 0.240 and 0.805, respectively, and the false-positive and false-negative rate was 0.195 and 0.760, respectively.
CONCLUSIONS
The DLST for mesalazine showed low sensitivity and high specificity, suggesting that it may be useful for the definitive diagnosis of allergy to mesalazine.
MeSH Terms
Figure
Reference
-
1. Matsuoka K, Watanabe M. Ulcerative colitis-recent advance in clinical practice and basic research. Nihon Shokakibyo Gakkai Zasshi. 2016; 113:407–412. PMID: 26947040.2. Ford AC, Achkar JP, Khan KJ, et al. Efficacy of 5-aminosalicylates in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2011; 106:601–616. PMID: 21407188.
Article3. Feagan BG, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012; 10:CD000543. DOI: 10.1002/14651858.CD000543.pub3. PMID: 23076889.
Article4. Sutherland L, Macdonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006; (2):CD000544. DOI: 10.1002/14651858.CD000544.pub2. PMID: 16625537.
Article5. Kobayashi K, Mukae M, Ogawa T, et al. 5-Aminosalicylic acid preparations for ulcerative colitis. Intestine. 2013; 17:127–132.6. Hanauer S, Schwartz J, Robinson M, et al. Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. Am J Gastroenterol. 1993; 88:1188–1197. PMID: 8338086.7. Iofel E, Chawla A, Daum F, Markowitz J. Mesalamine intolerance mimics symptoms of active inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2002; 34:73–76. PMID: 11753169.
Article8. Shimodate Y, Takanashi K, Waga E, Fujita T, Katsuki S, Nomura M. Exacerbation of bloody diarrhea as a side effect of mesalamine treatment of active ulcerative colitis. Case Rep Gastroenterol. 2011; 5:159–165. PMID: 21552438.
Article9. Nyfeler B, Pichler WJ. The lymphocyte transformation test for the diagnosis of drug allergy: sensitivity and specificity. Clin Exp Allergy. 1997; 27:175–181. PMID: 9061217.
Article10. Mantani N, Kogure T, Sakai S, et al. Incidence and clinical features of liver injury related to Kampo (Japanese herbal) medicine in 2,496 cases between 1979 and 1999: problems of the lymphocyte transformation test as a diagnostic method. Phytomedicine. 2002; 9:280–287. PMID: 12120808.
Article11. Watanabe M, Shibuya A, Satomichi A, et al. Diagnostic value of drug lymphocyte stimulation test and evaluation of diagnostic criteria for drug induced liver injury. Kanzo. 2001; 42:448–454.
Article12. Hagiwara K, Sato T, Akiyama O. Low specificity of lymphocyte stimulation test for methotrexate in the patients with rheumatoid arthritis. Allergy Pract. 2006; 26:46–50.13. Kawabata R, Koida M, Kanie S, Tanaka G, Ohuchida A, Yoshida T. DLST as a method for detecting TS-1-induced allergy. Gan To Kagaku Ryoho. 2006; 33:345–348.14. Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005; 100:1345–1353. PMID: 15929768.
Article15. Takikawa H, Sakisaka S, Aiso M, et al. Recent status of drug-induced liver injury: an analysis of 366 cases between 2002 and 2006. Kanzo. 2007; 48:517–521.
Article16. Abe K, Imaizumi H, Hayashi M, et al. Is the DLST valuable for diagnosis? Kan Tan Sui. 2014; 68:155–160.17. Eisenthal A, Eytan K, Brazowski E, Gitstein G, Greenberg R, Skornick Y. Effects of 5-FU on DNA synthesis and cytotoxicity of human lymphocytes induced by IL-2, TGF-beta3 and PGE2. Anticancer Res. 2009; 29:3925–3930. PMID: 19846930.18. Yamaguchi M. Drug-induced lymphocyte stimulation test. Medicina. 2015; 52:327–328.19. Shimizu H, Arai K, Tang J, Hosoi K, Funayama R. 5-Aminosalicylate intolerance causing exacerbation in pediatric ulcerative colitis. Pediatr Int. 2017; 59:583–587. PMID: 28063246.
Article20. Wada S, Kumagai H, Yokoyama K, et al. Mesalazine allergy in a boy with ulcerative colitis: clinical usefulness of mucosal biopsy criteria. Clin J Gastroenterol. 2016; 9:302–305. PMID: 27503129.
Article21. Fukushima T, Nakajima K, Henmi H, et al. Desensitization therapy for mesalazine-intolerant patients with inflammatory bowel disease. J Jpn Soc Coloproctol. 2014; 67:259–262.
Article22. Solem CA, Loftus EV Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005; 11:707–712. PMID: 16043984.
Article23. Henriksen M, Jahnsen J, Lygren I, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease: results from a prospective population-based study. Gut. 2008; 57:1518–1523. PMID: 18566104.
Article24. Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004; 59:809–820. PMID: 15230812.
Article25. Popple A, Williams J, Maxwell G, Gellatly N, Dearman RJ, Kimber I. The lymphocyte transformation test in allergic contact dermatitis: new opportunities. J Immunotoxicol. 2016; 13:84–91. PMID: 25655136.
Article26. Sugihara T, Koda M, Okamoto T, et al. The usefulness of second drug-induced lymphocyte stimulation tests (DLST). Kanzo. 2016; 57:571–576.
Article27. Fukuda H. Drug-induced lymphocyte stimulation test (DLST). Dr Salon. 2012; 56:35–39.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Allopurinol-Induced Fixed Drug Eruption Confirmed With a Lymphocyte Transformation Test
- Update on the diagnosis of drug allergy
- Mesalazine-induced Eosinophilic Pneumonia in a Patient with Crohn's Disease
- A Case of Gold Induced Hypersensitivity Pneumonitis Diagnosed by Lymphocyte Stimulation Test with Gold
- Acute eosinophilic pneumonia related to a mesalazine suppository