Yonsei Med J.
2018 Aug;59(6):754-759. 10.3349/ymj.2018.59.6.754.
Increased Thrombogenicity in Chronic Renal Failure in a Rat Model Induced by 5/6 Ablation/Infarction
- Affiliations
-
- 1Department of Neurology, Ewha Womans University College of Medicine, Seoul, Korea.
- 2Department of Neurology, Yonsei University College of Medicine, Seoul, Korea. jhheo@yuhs.ac
- 3Department of Anatomy, Ewha Womans University College of Medicine, Seoul, Korea.
- 4Ewha Institute of Convergence Medicine, Ewha Womans University, Seoul, Korea.
- KMID: 2415534
- DOI: http://doi.org/10.3349/ymj.2018.59.6.754
Abstract
- PURPOSE
Abnormalities in hemostasis and coagulation have been suggested in chronic renal failure (CRF). In this study, we compared processes of thrombus formation between rats with CRF and those with normal kidney function.
MATERIALS AND METHODS
CRF was induced by 5/6 ablation/infarction of the kidneys in Sprague-Dawley rats, and surviving rats after 4 weeks were used. Ferric chloride (FeCl3)-induced thrombosis in the carotid artery was induced to assess thrombus formation. Whole blood clot formation was evaluated using rotational thromboelastometry (ROTEM). Platelet aggregation was assessed with impedance platelet aggregometry.
RESULTS
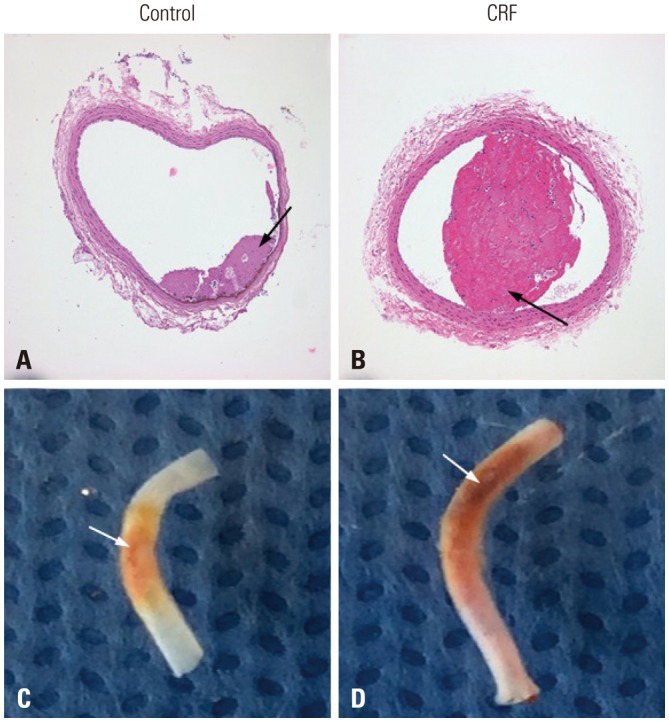
FeCl3-induced thrombus formation was initiated faster in the CRF group than in the control group (13.2±1.1 sec vs. 17.8±1.0 sec, p=0.027). On histological examination, the maximal diameters of thrombi were larger in the CRF group than in the control group (394.2±201.1 µm vs. 114.0±145.1 µm, p=0.039). In extrinsic pathway ROTEM, the CRF group showed faster clot initiation (clotting time, 59.0±7.3 sec vs. 72.8±5.0 sec, p=0.032) and increased clot growth kinetics (α angle, 84.8±0.2° vs. 82.0±0.6°, p=0.008), compared to the control group. Maximal platelet aggregation rate was higher in the CRF group than in the control group (58.2±0.2% vs. 44.6±1.2%, p=0.006).
CONCLUSION
Our study demonstrated that thrombogenicity is increased in rats with CRF. An activated extrinsic coagulation pathway may play an important role in increasing thrombogenicity in CRF.
MeSH Terms
Figure
Reference
-
1. Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005; 352:2049–2060. PMID: 15901858.
Article2. Kang YU, Kim MJ, Choi JS, Kim CS, Bae EH, Ma SK, et al. Concomitant impact of high-sensitivity C-reactive protein and renal dysfunction in patients with acute myocardial infarction. Yonsei Med J. 2014; 55:132–140. PMID: 24339298.
Article3. Kim K, Kim J, Ahn SH, Ha WS, Koo YJ, Kim DJ, et al. Histopathological findings of intracranial thrombi in nonbacterial thrombotic endocarditis. J Stroke. 2017; 19:367–369. PMID: 29037008.
Article4. Casserly LF, Dember LM. Thrombosis in end-stage renal disease. Semin Dial. 2003; 16:245–256. PMID: 12753687.
Article5. Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008; 19:135–140. PMID: 18032796.
Article6. Huang Y, Noble NA. PAI-1 as a target in kidney disease. Curr Drug Targets. 2007; 8:1007–1015. PMID: 17896952.
Article7. Hrafnkelsdóttir T, Ottosson P, Gudnason T, Samuelsson O, Jern S. Impaired endothelial release of tissue-type plasminogen activator in patients with chronic kidney disease and hypertension. Hypertension. 2004; 44:300–304. PMID: 15249548.
Article8. Lutz J, Menke J, Sollinger D, Schinzel H, Thürmel K. Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014; 29:29–40. PMID: 24132242.
Article9. Jalal DI, Chonchol M, Targher G. Disorders of hemostasis associated with chronic kidney disease. Semin Thromb Hemost. 2010; 36:34–40. PMID: 20391294.
Article10. Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009; 75:1145–1152. PMID: 19340094.
Article11. Rossini M, Naito T, Yang H, Freeman M, Donnert E, Ma LJ, et al. Sulodexide ameliorates early but not late kidney disease in models of radiation nephropathy and diabetic nephropathy. Nephrol Dial Transplant. 2010; 25:1803–1810. PMID: 20061322.
Article12. Bing P, Maode L, Li F, Sheng H. Expression of renal transforming growth factor-beta and its receptors in a rat model of chronic cyclosporine-induced nephropathy. Transplant Proc. 2006; 38:2176–2179. PMID: 16980035.13. Yang HC, Zuo Y, Fogo AB. Models of chronic kidney disease. Drug Discov Today Dis Models. 2010; 7:13–19. PMID: 21286234.
Article14. Lee SY, Shin JA, Kwon HM, Weiner ID, Han KH. Renal ischemiareperfusion injury causes intercalated cell-specific disruption of occludin in the collecting duct. Histochem Cell Biol. 2011; 136:637–647. PMID: 22048282.
Article15. Kwon I, Hong SY, Kim YD, Nam HS, Kang S, Yang SH, et al. Thrombolytic effects of the snake venom disintegrin saxatilin determined by novel assessment methods: a FeCl3-induced thrombosis model in mice. PLoS One. 2013; 8:e81165. PMID: 24260554.
Article16. Gava AL, Freitas FP, Balarini CM, Vasquez EC, Meyrelles SS. Effects of 5/6 nephrectomy on renal function and blood pressure in mice. Int J Physiol Pathophysiol Pharmacol. 2012; 4:167–173. PMID: 23071874.17. Erdely A, Wagner L, Muller V, Szabo A, Baylis C. Protection of wistar furth rats from chronic renal disease is associated with maintained renal nitric oxide synthase. J Am Soc Nephrol. 2003; 14:2526–2533. PMID: 14514730.
Article18. Degaspari S, Tzanno-Martins CB, Fujihara CK, Zatz R, Branco-Martins JP, Viel TA, et al. Altered KLOTHO and NF-κB-TNF-α signaling are correlated with nephrectomy-induced cognitive impairment in rats. PLoS One. 2015; 10:e0125271. PMID: 25961830.
Article19. Camenzind V, Bombeli T, Seifert B, Jamnicki M, Popovic D, Pasch T, et al. Citrate storage affects Thrombelastograph analysis. Anesthesiology. 2000; 92:1242–1249. PMID: 10781268.20. Meyer AS, Meyer MA, Sørensen AM, Rasmussen LS, Hansen MB, Holcomb JB, et al. Thrombelastography and rotational thromboelastometry early amplitudes in 182 trauma patients with clinical suspicion of severe injury. J Trauma Acute Care Surg. 2014; 76:682–690. PMID: 24553534.
Article21. Cruz MV, Luker JN, Carney BC, Brummel-Ziedins KE, Bravo MC, Orfeo T, et al. Reference ranges for rotational thromboelastometry in male Sprague Dawley rats. Thromb J. 2017; 15:31. PMID: 29299031.
Article22. Cook NS, Zerwes HG, Tapparelli C, Powling M, Singh J, Metternich R, et al. Platelet aggregation and fibrinogen binding in human, rhesus monkey, guinea-pig, hamster and rat blood: activation by ADP and a thrombin receptor peptide and inhibition by glycoprotein IIb/IIIa antagonists. Thromb Haemost. 1993; 70:531–539. PMID: 8259560.
Article23. Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. 1990; 60:269–280. PMID: 2087688.
Article24. Farrehi PM, Ozaki CK, Carmeliet P, Fay WP. Regulation of arterial thrombolysis by plasminogen activator inhibitor-1 in mice. Circulation. 1998; 97:1002–1008. PMID: 9529269.
Article25. Mangin P, Yap CL, Nonne C, Sturgeon SA, Goncalves I, Yuan Y, et al. Thrombin overcomes the thrombosis defect associated with platelet GPVI/FcRgamma deficiency. Blood. 2006; 107:4346–4353. PMID: 16391010.26. Molino D, De Lucia D, Gaspare De. Coagulation disorders in uremia. Semin Nephrol. 2006; 26:46–51. PMID: 16412826.
Article27. Johansson PI. Coagulation monitoring of the bleeding traumatized patient. Curr Opin Anaesthesiol. 2012; 25:235–241. PMID: 22193152.
Article28. Kaikita K, Takeya M, Ogawa H, Suefuji H, Yasue H, Takahashi K. Co-localization of tissue factor and tissue factor pathway inhibitor in coronary atherosclerosis. J Pathol. 1999; 188:180–188. PMID: 10398162.
Article29. Pawlak K, Tankiewicz J, Mysliwiec M, Pawlak D. Tissue factor/its pathway inhibitor system and kynurenines in chronic kidney disease patients on conservative treatment. Blood Coagul Fibrinolysis. 2009; 20:590–594. PMID: 19491664.
Article30. Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008; 359:938–949. PMID: 18753650.
Article31. Waki K, Hayashi A, Ikeda S, Ikeda S, Nagatsuka K, Honma Y, et al. Measuring platelet aggregation in dialysis patients with a whole blood aggregometer by the screen filtration pressure method. Ther Apher Dial. 2011; 15:203–206. PMID: 21426514.
Article32. Tay KH, Lip GY. What “drives” the link between the renin-angiotensin-aldosterone system and the prothrombotic state in hypertension? Am J Hypertens. 2008; 21:1278–1279. PMID: 19020509.
Article33. Zeck J, Schallheim J, Lew SQ, DePalma L. Whole blood platelet aggregation and release reaction testing in uremic patients. Biomed Res Int. 2013; 2013:486290. PMID: 23878808.
Article34. Chen GF, Baylis C. In vivo renal arginine release is impaired throughout development of chronic kidney disease. Am J Physiol Renal Physiol. 2010; 298:F95–F102. PMID: 19906948.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Experimental Model of Chronic Renal Failure in the Rat
- Myocardial Uptake of Tc-99m MDP in Chronic Renal Failure With Cardiomyopathy
- Clinical Experience of Hemodialysis on Three Cases of Renal Failure using Kill Type Artificial Kidney
- Three Cases of Renal Cell Carcinomas in Dialyzed Patients
- Non-medication Treatment of Atrial Fibrillation