J Periodontal Implant Sci.
2018 Jun;48(3):152-163. 10.5051/jpis.2018.48.3.152.
Oral tissue response to soft tissue expanders prior to bone augmentation: in vitro analysis and histological study in dogs
- Affiliations
-
- 1Department of Periodontology and Dental Research Institute, Translational Research Laboratory for Tissue Engineering (TTE), Seoul National University School of Dentistry, Seoul, Korea. periokoo@snu.ac.kr
- 2Osstem Implant Inc., Busan, Korea.
- KMID: 2414928
- DOI: http://doi.org/10.5051/jpis.2018.48.3.152
Abstract
- PURPOSE
To determine whether the swelling and mechanical properties of osmotic self-inflating expanders allow or not the induction of intraoral soft tissue expansion in dogs.
METHODS
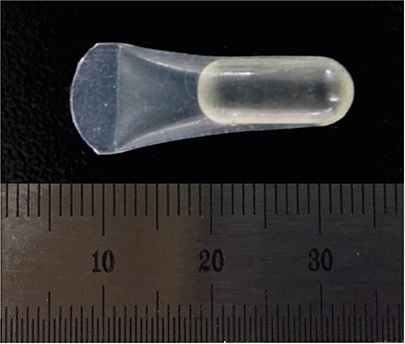
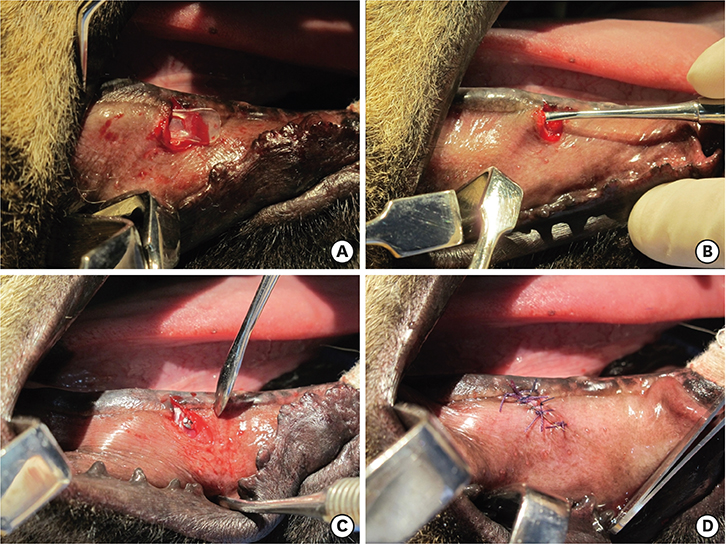
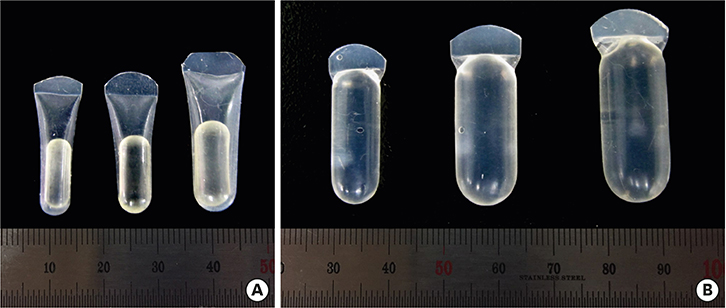
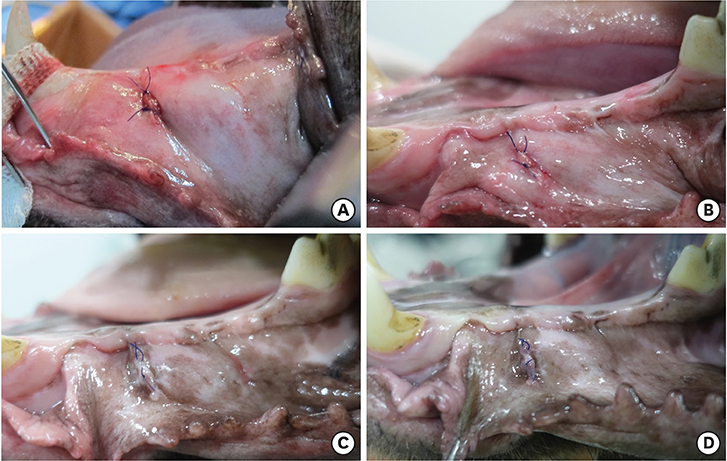
Three different volumes (0.15, 0.25, and 0.42 mL; referred to respectively as the S, M, and L groups) of soft tissue expanders (STEs) consisting of a hydrogel core coated with a silicone-perforated membrane were investigated in vitro to assess their swelling behavior (volume swelling ratio) and mechanical properties (tensile strength, tensile strain). For in vivo investigations, the STEs were subperiosteally inserted for 4 weeks in dogs (n=5). Soft tissue expansion was clinically monitored. Histological analyses included the examination of alveolar bone underneath the expanders and thickness measurements of the surrounding fibrous capsule.
RESULTS
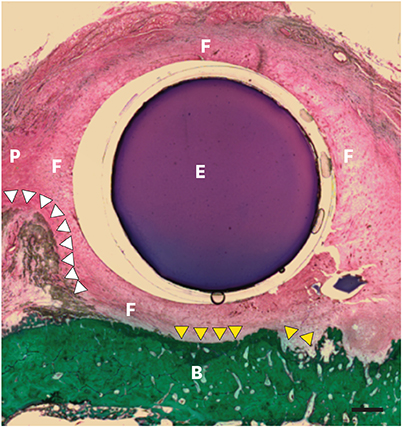
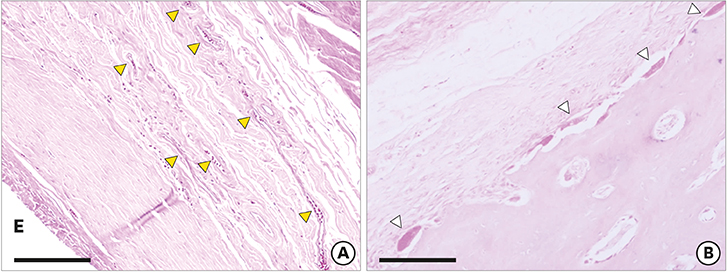
The volume swelling ratio of all STEs did not exceed 5.2. In tensile mode, the highest mean strain was registered for the L group (98.03±0.3 g/cm), whereas the lowest mean value was obtained in the S group (81.3±0.1 g/cm), which was a statistically significant difference (P < 0.05). In addition, the S and L groups were significantly different in terms of tensile strength (1.5±0.1 g/cm for the S group and 2.2±0.1 g/cm for the L group, P < 0.05). Clinical monitoring showed successful dilatation of the soft tissues without signs of inflammation up to 28 days. The STEs remained volumetrically stable, with a mean diameter in vivo of 6.98 mm, close to the in vitro post-expansion findings (6.69 mm). Significant histological effects included highly vascularized collagen-rich fibrous encapsulation of the STEs, with a mean thickness of 0.67±0.12 mm. The bone reaction consisted of resorption underneath the STEs, while apposition was observed at their edges.
CONCLUSIONS
The swelling and mechanical properties of the STEs enabled clinically successful soft tissue expansion. A tissue reaction consisting of fibrous capsule formation and bone loss were the main histological events.
MeSH Terms
Figure
Reference
-
1. Cordaro L, Amadé DS, Cordaro M. Clinical results of alveolar ridge augmentation with mandibular block bone grafts in partially edentulous patients prior to implant placement. Clin Oral Implants Res. 2002; 13:103–111.
Article2. Sonick M, Hwang D. Tooth extraction—an opportunity for site preservation. Contemp Esthetics. 2007; 11:36–41.3. Rocchietta I, Fontana F, Simion M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: a systematic review. J Clin Periodontol. 2008; 35:Suppl. 203–215.
Article4. Esposito M, Grusovin MG, Felice P, Karatzopoulos G, Worthington HV, Coulthard P. Interventions for replacing missing teeth: horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database Syst Rev. 2009; 7:CD003607.
Article5. Uckan S, Dolanmaz D, Kalayci A, Cilasun U. Distraction osteogenesis of basal mandibular bone for reconstruction of the alveolar ridge. Br J Oral Maxillofac Surg. 2002; 40:393–396.
Article6. Mertens C, Thiele O, Engel M, Seeberger R, Hoffmann J, Freier K. The use of self-inflating soft tissue expanders prior to bone augmentation of atrophied alveolar ridges. Clin Implant Dent Relat Res. 2015; 17:44–51.
Article7. Asa'ad F, Rasperini G, Pagni G, Rios HF, Giannì AB. Pre-augmentation soft tissue expansion: an overview. Clin Oral Implants Res. 2016; 27:505–522.8. von See C, Rücker M, Schumann P, Goetz F, Wefstaedt P, Nolte I, et al. Micro-computed tomography and histologic evaluation of the interface of hydrogel expander and underlying bone: influence of pressure distributors on bone resorption. J Oral Maxillofac Surg. 2010; 68:2179–2184.
Article9. Abrahamsson P, Isaksson S, Gordh M, Andersson G. Periosteal expansion of rabbit mandible with an osmotic self-inflatable expander. Scand J Plast Reconstr Surg Hand Surg. 2009; 43:121–125.
Article10. Kaner D, Friedmann A. Soft tissue expansion with self-filling osmotic tissue expanders before vertical ridge augmentation: a proof of principle study. J Clin Periodontol. 2011; 38:95–101.
Article11. Kaner D, Zhao H, Terheyden H, Friedmann A. Improvement of microcirculation and wound healing in vertical ridge augmentation after pre-treatment with self-inflating soft tissue expanders - a randomized study in dogs. Clin Oral Implants Res. 2015; 26:720–724.
Article12. Tominaga K, Matsuo T, Kuga Y, Mizuno A. An animal model for subperiosteal tissue expansion. J Oral Maxillofac Surg. 1993; 51:1244–1249.
Article13. Abrahamsson P, Isaksson S, Andersson G. Guided bone generation in a rabbit mandible model after periosteal expansion with an osmotic tissue expander. Clin Oral Implants Res. 2011; 22:1282–1288.
Article14. Sarvestani AS, Xu W, He X, Jabbari E. Gelation and degradation characteristics of in situ photo-crosslinked poly (l-lactide-co-ethylene oxide-co-fumarate) hydrogels. Polymer (Guildf). 2007; 48:7113–7120.
Article15. Zhu Y, Czernuszka JT. Inter-penetrating polymer network hydrogel tissue expanders with controlled expansion and anisotropic properties. J Med Bioeng. 2015; 4:86–92.
Article16. Neumann CG. The expansion of an area of skin by progressive distention of a subcutaneous balloon; use of the method for securing skin for subtotal reconstruction of the ear. Plast Reconstr Surg (1946). 1957; 19:124–130.
Article17. Garner J, Davidson D, Eckert GJ, Barco CT, Park H, Park K. Reshapable polymeric hydrogel for controlled soft-tissue expansion: in vitro and in vivo evaluation. J Control Release. 2017; 262:201–211.
Article18. Wiese KG. Osmotically induced tissue expansion with hydrogels: a new dimension in tissue expansion? A preliminary report. J Craniomaxillofac Surg. 1993; 21:309–313.
Article19. Wiese KG, Vogel M, Guthoff R, Gundlach KK. Treatment of congenital anophthalmos with self-inflating polymer expanders: a new method. J Craniomaxillofac Surg. 1999; 27:72–76.
Article20. Wiese KG, Heinemann DE, Ostermeier D, Peters JH. Biomaterial properties and biocompatibility in cell culture of a novel self-inflating hydrogel tissue expander. J Biomed Mater Res. 2001; 54:179–188.
Article21. Lin S, Sangaj N, Razafiarison T, Zhang C, Varghese S. Influence of physical properties of biomaterials on cellular behavior. Pharm Res. 2011; 28:1422–1430.
Article22. Werfully S, Areibi G, Toner M, Bergquist J, Walker J, Renvert S, et al. Tensile strength, histological and immunohistochemical observations of periodontal wound healing in the dog. J Periodontal Res. 2002; 37:366–374.
Article23. Sato T, Hara T, Mori S, Shirai H, Minagi S. Threshold for bone resorption induced by continuous and intermittent pressure in the rat hard palate. J Dent Res. 1998; 77:387–392.
Article24. Uijlenbroek HJ, Liu Y, He JF, Visscher C, van Waas MA, Wismeyer D. Expanding soft tissue with Osmed tissue expanders in the goat maxilla. Clin Oral Implants Res. 2011; 22:121–128.
Article25. Uijlenbroek HJ, Liu Y, Wismeijer D. Soft tissue expansion: principles and inferred intraoral hydrogel tissue expanders. Dent Oral Craniofac Res. 2015; 1:178–185.
Article26. Poeppl N, Schreml S, Lichtenegger F, Lenich A, Eisenmann-Klein M, Prantl L. Does the surface structure of implants have an impact on the formation of a capsular contracture? Aesthetic Plast Surg. 2007; 31:133–139.
Article27. Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011; 32:6692–6709.
Article28. Austad ED, Pasyk KA, McClatchey KD, Cherry GW. Histomorphologic evaluation of guinea pig skin and soft tissue after controlled tissue expansion. Plast Reconstr Surg. 1982; 70:704–710.
Article29. Argenta LC, Marks MW, Pasyk KA. Advances in tissue expansion. Clin Plast Surg. 1985; 12:159–171.
Article30. Abrahamsson P, Wälivaara DÅ, Isaksson S, Andersson G. Periosteal expansion before local bone reconstruction using a new technique for measuring soft tissue profile stability: a clinical study. J Oral Maxillofac Surg. 2012; 70:e521–e530.
Article31. Johnson TM, Lowe L, Brown MD, Sullivan MJ, Nelson BR. Histology and physiology of tissue expansion. J Dermatol Surg Oncol. 1993; 19:1074–1078.
Article32. Kessler P, Bumiller L, Schlegel A, Birkholz T, Neukam FW, Wiltfang J. Dynamic periosteal elevation. Br J Oral Maxillofac Surg. 2007; 45:284–287.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Self-inflating oral tissue expander for ridge augmentation in the severely atrophic mandible
- Effects of implant collar design on marginal bone and soft tissue
- A Case Report of Progressive Hemifacial Atrophy
- Tissue Reaction in Response to Augmentation of Soft Tissue Defects According to the Dermis Graft Materials in Rabbits
- Regeneration of total tissue using alveolar ridge augmentation with soft tissue substitute on periodontally compromised extraction sites: case report