J Korean Soc Transplant.
2018 Jun;32(2):13-25. 10.4285/jkstn.2018.32.2.13.
Results of Questionnaire Survey of Current Immune Monitoring Practice of Transplant Clinicians and Clinical Pathologists in Korea: Basis for Establishment of Harmonized Immune Monitoring Guidelines
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. eskang@skku.edu
- 2Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2414444
- DOI: http://doi.org/10.4285/jkstn.2018.32.2.13
Abstract
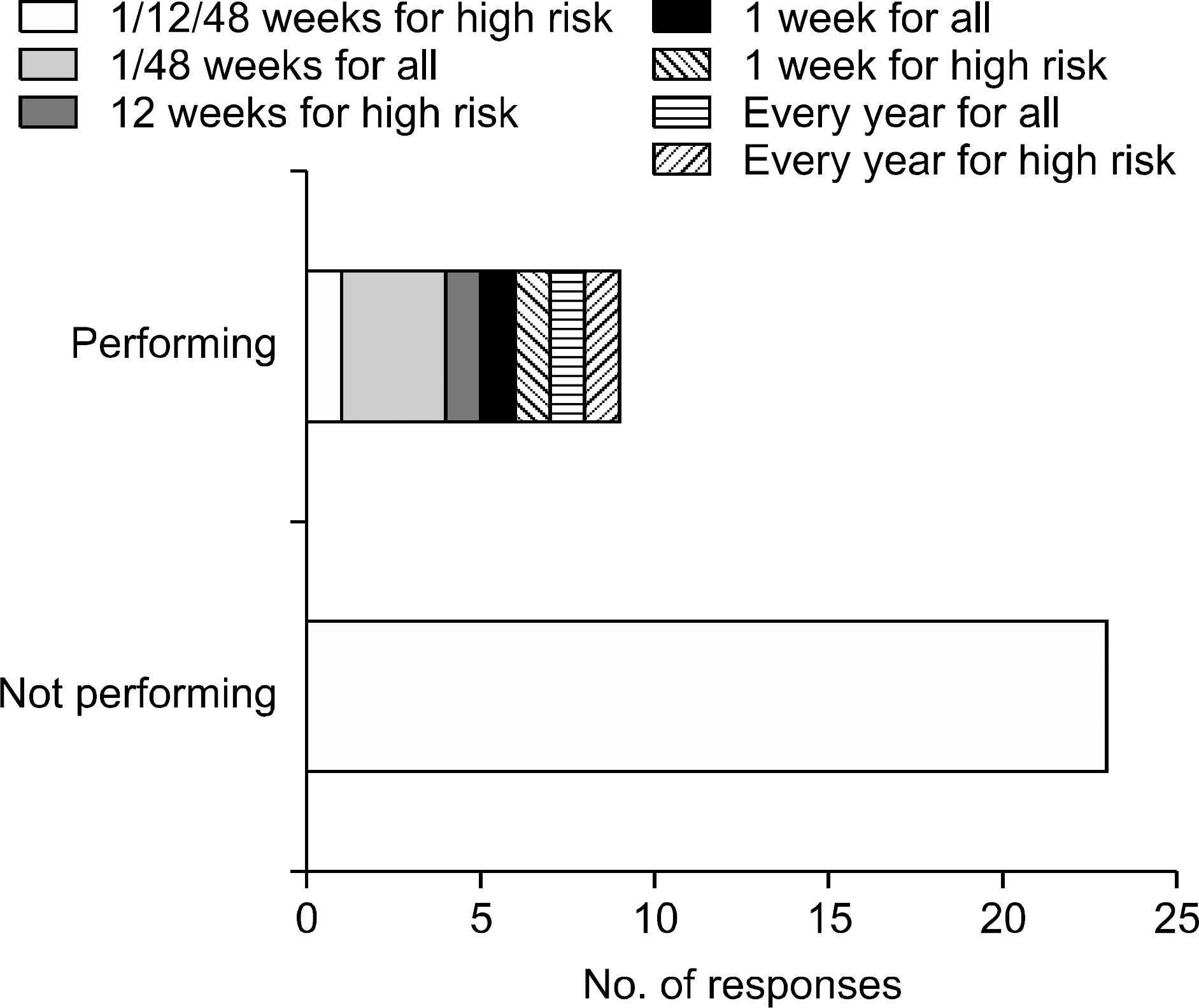
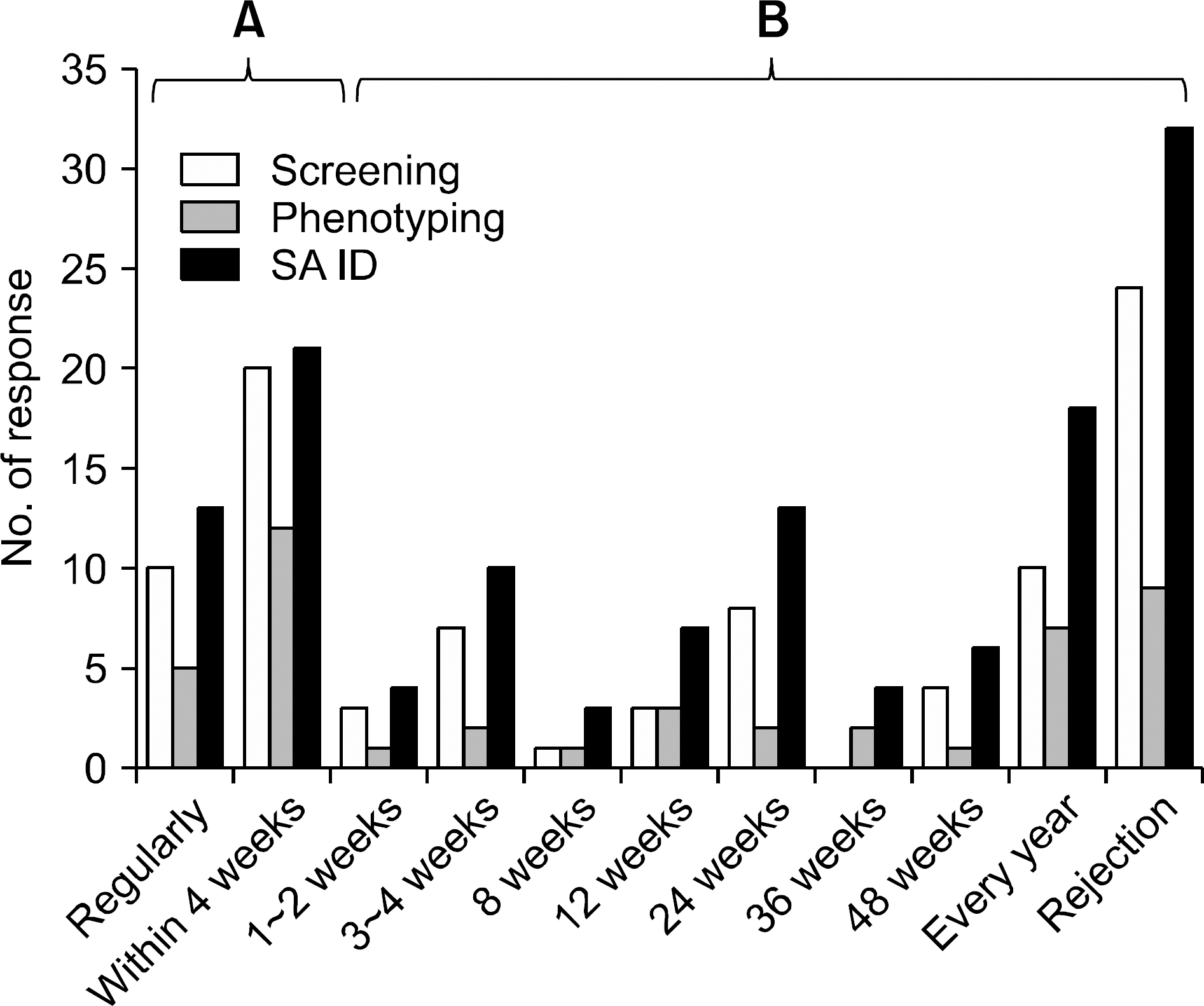
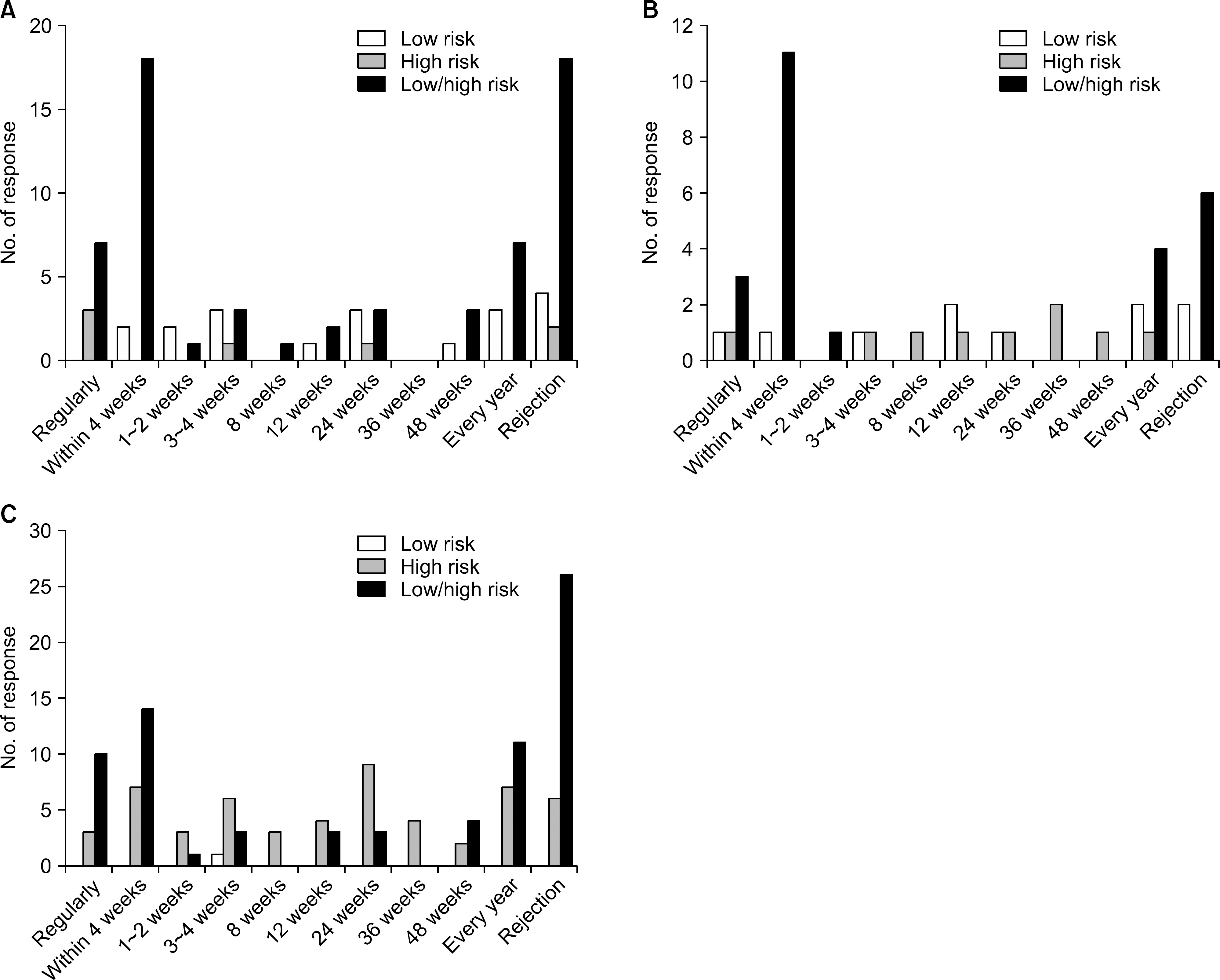
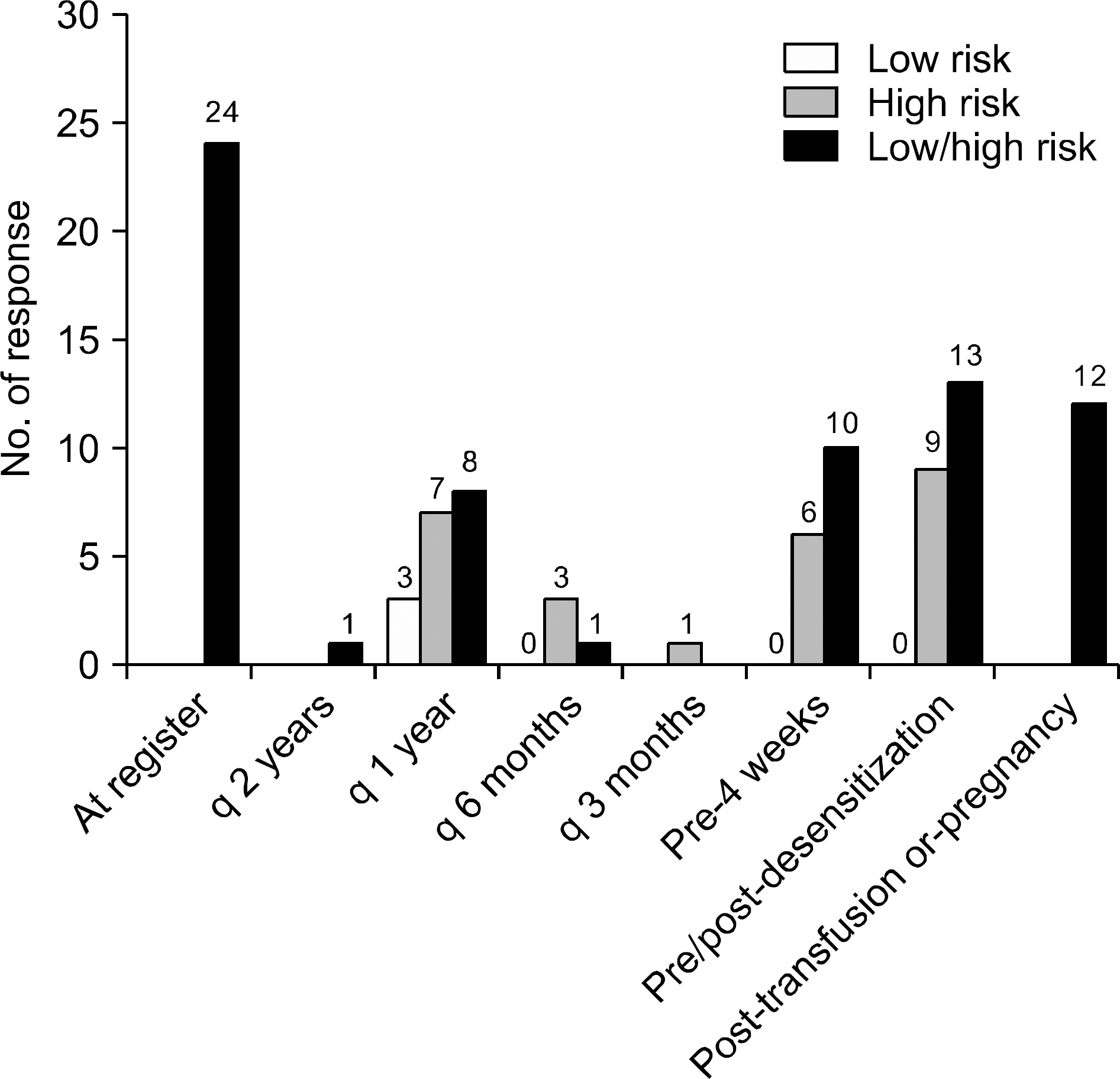
- Detection of significant alloimmune response, which affects graft function and survival by effective immune monitoring, is critical for treatment decision making. However, there is no consensus regarding immune monitoring (IM) for kidney transplantation (flow KT) in Korea. The IM protocol may be affected by the level of immunological risk, the methods of desensitization and the availabilities of resources such as laboratory support and cost of tests. Questionnaire surveys designed to identify the current practices regarding immune monitoring of KT among transplant clinicians and clinical pathologists in Korea and eventually provide a basis for the establishment of harmonized immune monitoring guidelines in KT were administered as part of a Korean Society for Transplantation Sponsored Research Project. The survey results revealed significant variations in IM protocols and interpretation of tests affecting treatment decisions between institutes. Moreover, the results revealed a need to expand the histocompatibility tests into high resolution HLA typing in multiple loci and non-HLA antibody tests that facilitate the epitope analysis and eventually virtual crossmatching. The results of the questionnaire survey from clinical pathologists are addressing the urgent need for the standardization of interpretation and harmonization of results reporting in single antigen bead based HLA antibody identification. Finally, communication between clinicians and clinical pathologists to meet the clinical expectations regarding various immune monitoring tests is needed.
MeSH Terms
Figure
Reference
-
1). Hart A., Smith JM., Skeans MA., Gustafson SK., Stewart DE., Cherikh WS, et al. OPTN/SRTR 2015 Annual Data Report: kidney. Am J Transplant. 2017. 17(Suppl 1):21–116.
Article2). Bartel G., Wahrmann M., Regele H., Kikic Z., Fischer G., Druml W, et al. Peritransplant immunoadsorption for positive crossmatch deceased donor kidney transplantation. Am J Transplant. 2010. 10:2033–42.
Article3). Morath C., Beimler J., Opelz G., Scherer S., Schmidt J., Macher-Goeppinger S, et al. Living donor kidney transplantation in crossmatch-positive patients enabled by peritransplant immunoadsorption and anti-CD20 therapy. Transpl Int. 2012. 25:506–17.
Article4). Morath C., Beimler J., Opelz G., Ovens J., Scherer S., Schmidt J, et al. An integrative approach for the transplantation of high-risk sensitized patients. Transplantation. 2010. 90:645–53.
Article5). Pei R., Lee JH., Shih NJ., Chen M., Terasaki PI. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation. 2003. 75:43–9.
Article6). Taylor CJ., Kosmoliaptsis V., Summers DM., Bradley JA. Back to the future: application of contemporary technology to long-standing questions about the clinical relevance of human leukocyte antigen-specific alloantibodies in renal transplantation. Hum Immunol. 2009. 70:563–8.
Article7). Cai J., Terasaki PI. Post-transplantation antibody monitoring and HLA antibody epitope identification. Curr Opin Immunol. 2008. 20:602–6.
Article8). Kosmoliaptsis V., Bradley JA., Sharples LD., Chaudhry A., Key T., Goodman RS, et al. Predicting the immunogenicity of human leukocyte antigen class I alloantigens using structural epitope analysis determined by HLAMatchmaker. Transplantation. 2008. 85:1817–25.
Article9). Duquesnoy RJ. HLAMatchmaker: a molecularly based algorithm for histocompatibility determination. I. Description of the algorithm. Hum Immunol. 2002. 63:339–52.
Article10). Dunn TB., Noreen H., Gillingham K., Maurer D., Ozturk OG., Pruett TL, et al. Revisiting traditional risk factors for rejection and graft loss after kidney transplantation. Am J Transplant. 2011. 11:2132–43.
Article11). Billen EV., Christiaans MH., Doxiadis II., Voorter CE., van den Berg-Loonen EM. HLA-DP antibodies before and after renal transplantation. Tissue Antigens. 2010. 75:278–85.
Article12). Dragun D., Muller DN., Brasen JH., Fritsche L., Nieminen-Kelha M., Dechend R, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005. 352:558–69.
Article13). Reinsmoen NL., Lai CH., Heidecke H., Haas M., Cao K., Ong G, et al. Anti-angiotensin type 1 receptor antibodies associated with antibody mediated rejection in donor HLA antibody negative patients. Transplantation. 2010. 90:1473–7.
Article14). Reinsmoen NL., Lai CH., Mirocha J., Cao K., Ong G., Naim M, et al. Increased negative impact of donor HLA-specific together with non-HLA-specific antibodies on graft outcome. Transplantation. 2014. 97:595–601.
Article15). Ming Y., Hu J., Luo Q., Ding X., Luo W., Zhuang Q, et al. Acute antibody-mediated rejection in presence of MICA-DSA and successful renal re-transplant with negative-MICA virtual crossmatch. PLoS One. 2015. 10:e0127861.
Article16). O Broin P., Hayde N., Bao Y., Ye B., Calder RB., de Boccardo G, et al. A pathogenesis-based transcript signature in donor-specific antibody-positive kidney transplant patients with normal biopsies. Genom Data. 2014. 2:357–60.17). Kurian SM., Velazquez E., Thompson R., Whisenant T., Rose S., Riley N, et al. Orthogonal comparison of molecular signatures of kidney transplants with subclinical and clinical acute rejection: equivalent performance is agnostic to both technology and platform. Am J Transplant. 2017. 17:2103–16.
Article18). Choi JW., Kim YH., Oh JW. Comparative analyses of signature genes in acute rejection and operational tolerance. Immune Netw. 2017. 17:237–49.
Article19). Loupy A., Haas M., Solez K., Racusen L., Glotz D., Seron D, et al. The Banff 2015 Kidney Meeting Report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant. 2017. 17:28–41.
Article20). Haas M., Loupy A., Lefaucheur C., Roufosse C., Glotz D., Seron D, et al. The Banff 2017 Kidney Meeting report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018. 18:293–307.
Article21). Wiebe C., Gibson IW., Blydt-Hansen TD., Karpinski M., Ho J., Storsley LJ, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012. 12:1157–67.
Article22). Gloor JM., Cosio FG., Rea DJ., Wadei HM., Winters JL., Moore SB, et al. Histologic findings one year after positive crossmatch or ABO blood group incompatible living donor kidney transplantation. Am J Transplant. 2006. 6:1841–7.
Article23). Burns JM., Cornell LD., Perry DK., Pollinger HS., Gloor JM., Kremers WK, et al. Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant. 2008. 8:2684–94.
Article24). Amico P., Honger G., Mayr M., Steiger J., Hopfer H., schaub S. Clinical relevance of pre-transplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation. 2009. 87:1681–8.
Article25). Hirai T., Kohei N., Omoto K., Ishida H., Tanabe K. Significance of low-level DSA detected by solid-phase assay in association with acute and chronic antibody-mediated rejection. Transpl Int. 2012. 25:925–34.
Article26). Tian J., Li D., Alberghini TV., Rewinski M., Guo N., Bow LM. Pre-transplant low level HLA antibody shows a composite poor outcome in long-term outcome of renal transplant recipients. Ren Fail. 2015. 37:198–202.
Article27). Tait BD., Susal C., Gebel HM., Nickerson PW., Zachary AA., Claas FH, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013. 95:19–47.
Article28). Korean Network for Organ Sharing (KONOS). 2017 Annual Data Report [Internet]. Seoul: KONOS;2017. Available from:. http://konos.go.kr.29). Oh EJ., Park H., Park KU., Kang ES., Kim HS., Song EY. Interlaboratory Comparison of the results of lifecodes LSA class I and class II single antigen kits for human leukocyte antigen antibody detection. Ann Lab Med. 2015. 35:321–8.
Article30). Yu S., Kang ES., Park MH. A questionnaire survey of HLA crossmatch tests in Korea (2015). Lab Med Online. 2017. 7:147–56.
Article31). Dunn TB., Noreen H., Gillingham K., Maurer D., Ozturk OG., Pruett TL, et al. Revisiting traditional risk factors for rejection and graft loss after kidney transplantation. Am J Transplant. 2011. 11:2132–43.
Article32). Lee H., Min JW., Kim JI., Moon IS., Park KH., Yang CW, et al. Clinical significance of HLA-DQ antibodies in the development of chronic antibody-mediated rejection and allograft failure in kidney transplant recipients. Medicine (Baltimore). 2016. 95:e3094.
Article33). Bachelet T., Martinez C., Del Bello A., Couzi L., Kejji S., Guidicelli G, et al. Deleterious impact of donor-specific anti-HLA antibodies toward HLA-Cw and HLA-DP in kidney transplantation. Transplantation. 2016. 100:159–66.
Article34). Tagliamacco A., Cioni M., Comoli P., Ramondetta M., Brambilla C., Trivelli A, et al. DQ molecules are the principal stimulators of de novo donor-specific antibodies in nonsensitized pediatric recipients receiving a first kidney transplant. Transpl Int. 2014. 27:667–73.35). Edathodu J., Varghese B., Alrajhi AA., Shoukri M., Nazmi A., Elgamal H, et al. Diagnostic potential of interferon-gamma release assay to detect latent tuberculosis infection in kidney transplant recipients. Transpl Infect Dis. 2017. 19:e12675.
Article36). Banas B., Steubl D., Renders L., Chittka D., Banas MC., Wekerle T, et al. Clinical validation of a novel enzyme-linked immunosorbent spot assay-based in vitro diagnostic assay to monitor cytomegalovirus-specific cell-mediated immunity in kidney transplant recipients: a multicenter, longitudinal, prospective, observational study. Transpl Int. 2018. 31:436–50.37). Wang Z., Liu X., Lu P., Han Z., Tao J., Wang J, et al. Performance of the ImmuKnow assay in differentiating infection and acute rejection after kidney transplantation: a meta-analysis. Transplant Proc. 2014. 46:3343–51.
Article38). Potena L., Gaudenzi A., Chiereghin A., Borgese L., Brighenti A., Piccirilli G, et al. Quantiferon monitor assay identifies over-immunosuppressed patients with adverse outcomes after heart transplantation: towards the definition of a phenotype of immune frailty. J Heart Lung Transplant. 2018. 37(4 Suppl):S19–20.
Article39). Haas M., Sis B., Racusen LC., Solez K., Glotz D., Colvin RB, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014. 14:272–83.
Article40). Marsh SG., Albert ED., Bodmer WF., Bontrop RE., Dupont B., Erlich HA, et al. An update to HLA nomenclature, 2010. Bone Marrow Transplant. 2010. 45:846–8.
Article41). Amico P., Hirt-Minkowski P., Honger G., Gurke L., Mihatsch MJ., Steiger J, et al. Risk stratification by the virtual crossmatch: a prospective study in 233 renal transplantations. Transpl Int. 2011. 24:560–9.
Article42). Kosmoliaptsis V., Bradley JA., Peacock S., Chaudhry AN., Taylor CJ. Detection of immunoglobulin G human leukocyte antigen-specific alloantibodies in renal transplant patients using single-antigen-beads is compromised by the presence of immunoglobulin M human leukocyte antigen-specific alloantibodies. Transplantation. 2009. 87:813–20.
Article43). Schnaidt M., Weinstock C., Jurisic M., Schmid-Horch B., Ender A., Wernet D. HLA antibody specification using single-antigen beads: a technical solution for the prozone effect. Transplantation. 2011. 92:510–5.44). Weinstock C., Schnaidt M. The complement-mediated prozone effect in the Luminex single-antigen bead assay and its impact on HLA antibody determination in patient sera. Int J Immunogenet. 2013. 40:171–7.
Article45). Moreno Gonzales MA., Mitema DG., Smith BH., Schinstock CA., Stegall MD., Wakefield LL, et al. Comparison between total IgG, C1q, and C3d single antigen bead assays in detecting class I complement-binding anti-HLA antibodies. Transplant Proc. 2017. 49:2031–5.
Article46). Tait BD. Detection of HLA antibodies in organ transplant recipients-triumphs and challenges of the solid phase bead assay. Front Immunol. 2016. 7:570.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunologic monitoring in kidney transplant recipients
- What anesthesiologists ask to know and should know about the neuromuscular monitoring: an updated review
- Clinical practice guidelines for intraoperative neurophysiological monitoring: 2020 update
- Investigation and Standardization on Current Practice of Renal Transplant Pathology in Korea
- SARS-CoV-2 vaccine-elicited immune responses in solid organ transplant recipients