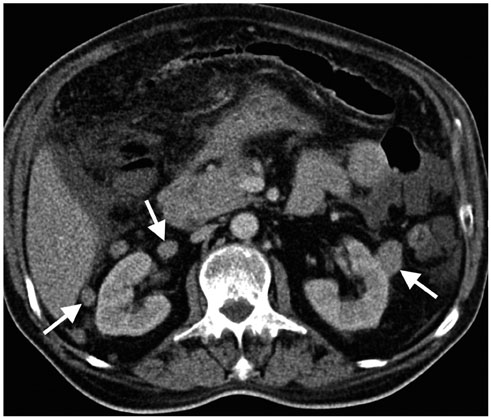
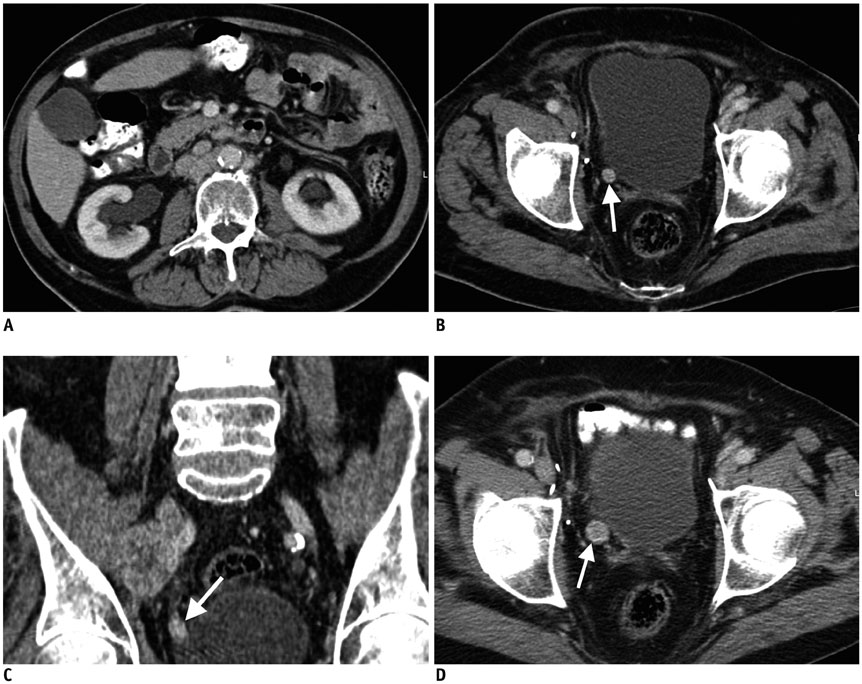
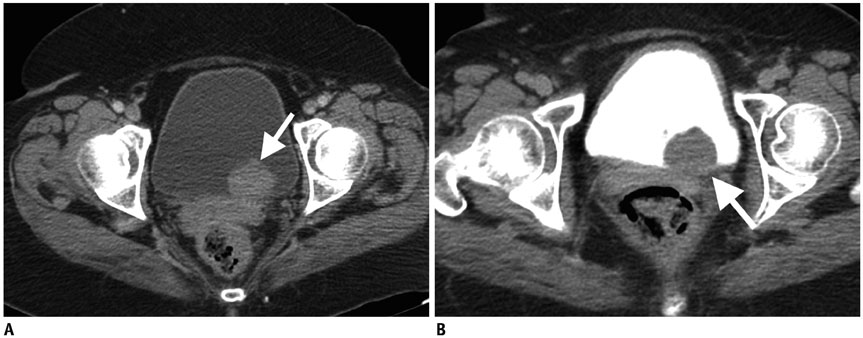
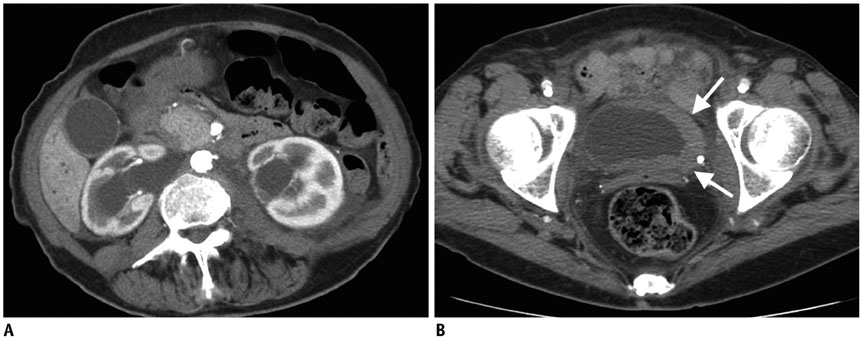
Korean J Radiol.
2018 Aug;19(4):742-751. 10.3348/kjr.2018.19.4.742.
Secondary Tumors of the Urinary System: An Imaging Conundrum
- Affiliations
-
- 1Department of Radiology, Hacettepe University, School of Medicine, Ankara 06230, Turkey. ruhionur@yahoo.com
- KMID: 2413703
- DOI: http://doi.org/10.3348/kjr.2018.19.4.742
Abstract
- Imaging features of metastases to the urinary system may closely mimic primary urinary tract tumors, and differential diagnosis by imaging alone may be problematic or even impossible in some cases. The main purpose of this article was to familiarize radiologists with imaging findings of metastasis to the urinary system on cross-sectional imaging, with an emphasis on abdominal and pelvic computed tomography and magnetic resonance imaging. In addition, we review the clinical importance and implications of metastases to the urinary tract and provide information on diagnostic work-ups.
Keyword
- Urinary system; Metastasis; Kidney; Ureter; Bladder; Urethra; CT; MRI
MeSH Terms
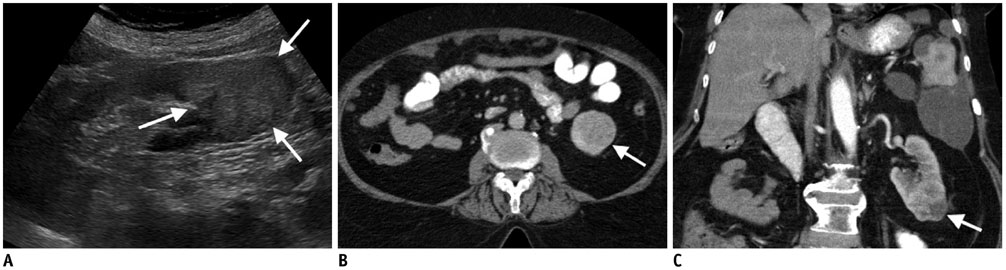
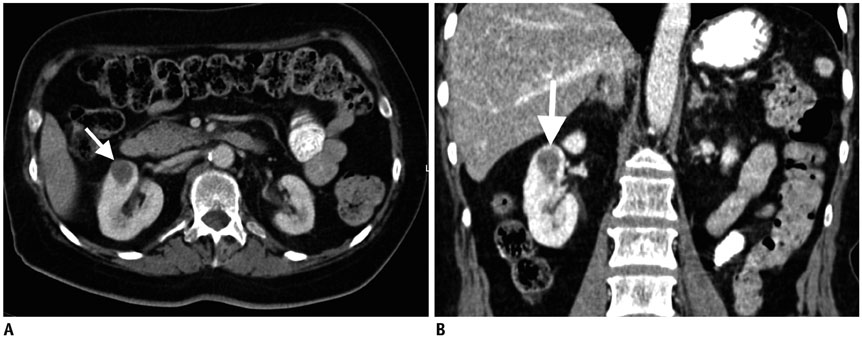
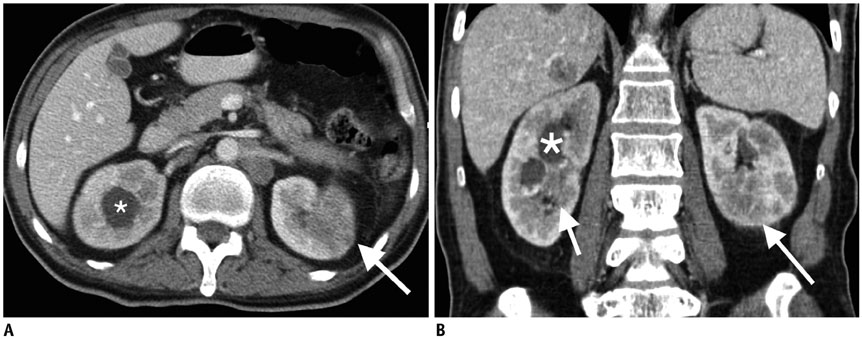
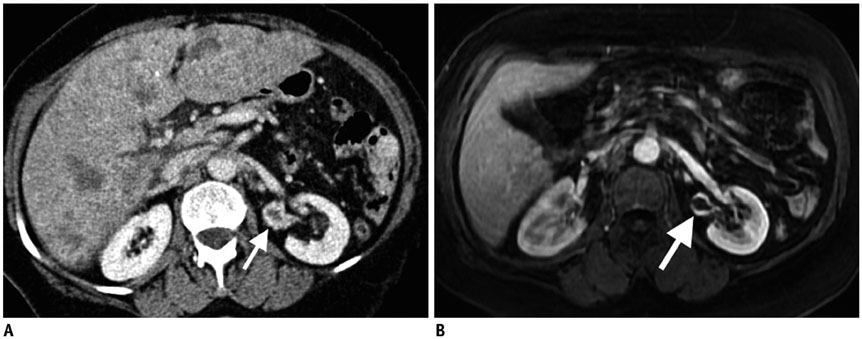
Figure
Reference
-
1. Arvind NK, Singh O, Gupta S, Ali Q. Ureteral metastasis as the presenting manifestation of pancreatic carcinoma. Rev Urol. 2013; 15:124–130.2. Doo SW, Kim WB, Kim BK, Yang WJ, Yoon JH, Jin SY, et al. Metastasis of renal cell carcinoma to the bladder. Korean J Urol. 2013; 54:69–72.
Article3. Zhou C, Urbauer DL, Fellman BM, Tamboli P, Zhang M, Matin SF, et al. Metastases to the kidney: a comprehensive analysis of 151 patients from a tertiary referral centre. BJU Int. 2016; 117:775–782.
Article4. Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950; 3:74–85.5. Bracken RB, Chica G, Johnson DE, Luna M. Secondary renal neoplasms: an autopsy study. South Med J. 1979; 72:806–807.6. Bates AW, Baithun SI. The significance of secondary neoplasms of the urinary and male genital tract. Virchows Arch. 2002; 440:640–647.
Article7. Patel U, Ramachandran N, Halls J, Parthipun A, Slide C. Synchronous renal masses in patients with a nonrenal malignancy: incidence of metastasis to the kidney versus primary renal neoplasia and differentiating features on CT. AJR Am J Roentgenol. 2011; 197:W680–W686.
Article8. Choyke PL, White EM, Zeman RK, Jaffe MH, Clark LR. Renal metastases: clinicopathologic and radiologic correlation. Radiology. 1987; 162:359–363.
Article9. Prasad SR, Dalrymple NC, Surabhi VR. Cross-sectional imaging evaluation of renal masses. Radiol Clin North Am. 2008; 46:95–111. vi–vii.
Article10. Sánchez-Ortiz RF, Madsen LT, Bermejo CE, Wen S, Shen Y, Swanson DA, et al. A renal mass in the setting of a nonrenal malignancy: when is a renal tumor biopsy appropriate? Cancer. 2004; 101:2195–2201.11. Adamy A, Von Bodman C, Ghoneim T, Favaretto RL, Bernstein M, Russo P. Solitary, isolated metastatic disease to the kidney: Memorial Sloan-Kettering Cancer Center experience. BJU Int. 2011; 108:338–342.
Article12. Olsson CA, Moyer JD, Laferte RO. Pulmonary cancer metastatic to the kidney--a common renal neoplasm. J Urol. 1971; 105:492–496.
Article13. Klinger ME. Secondary tumors of the genito-urinary tract. J Urol. 1951; 65:144–153.
Article14. Mitnick JS, Bosniak MA, Rothberg M, Megibow AJ, Raghavendra BN, Subramanyam BR. Metastatic neoplasm to the kidney studied by computed tomography and sonography. J Comput Assist Tomogr. 1985; 9:43–49.
Article15. Bailey JE, Roubidoux MA, Dunnick NR. Secondary renal neoplasms. Abdom Imaging. 1998; 23:266–274.
Article16. Bhatt GM, Bernardino ME, Graham SD Jr. CT diagnosis of renal metastases. J Comput Assist Tomogr. 1983; 7:1032–1034.
Article17. Vikram R, Beland MD, Blaufox MD, Moreno CC, Gore JL, Harvin HJ, et al. ACR appropriateness criteria renal cell carcinoma staging. J Am Coll Radiol. 2016; 13:518–525.
Article18. Sun MR, Pedrosa I. Magnetic resonance imaging of renal masses. Semin Ultrasound CT MR. 2009; 30:326–351.
Article19. Ferrozzi F, Bova D, Campodonico F. Computed tomography of renal metastases. Semin Ultrasound CT MR. 1997; 18:115–121.
Article20. Maurer MH, Härmä KH, Thoeny H. Diffusion-weighted genitourinary imaging. Radiol Clin North Am. 2017; 55:393–411.
Article21. Thoeny HC, De Keyzer F, Oyen RH, Peeters RR. Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology. 2005; 235:911–917.
Article22. Heller MT, Haarer KA, Thomas E, Thaete FL. Neoplastic and proliferative disorders of the perinephric space. Clin Radiol. 2012; 67:e31–e41.
Article23. Surabhi VR, Menias C, Prasad SR, Patel AH, Nagar A, Dalrymple NC. Neoplastic and non-neoplastic proliferative disorders of the perirenal space: cross-sectional imaging findings. Radiographics. 2008; 28:1005–1017.
Article24. Grützner G, Jungblut RM. [Perirenal metastasis of a malignant melanoma in a young child]. Aktuelle Radiol. 1993; 3:372–374.25. Wilbur AC, Turk JN, Capek V. Perirenal metastases from lung cancer: CT diagnosis. J Comput Assist Tomogr. 1992; 16:589–591.26. Gore RM, Balfe DM, Aizenstein RI, Silverman PM. The great escape: interfascial decompression planes of the retroperitoneum. AJR Am J Roentgenol. 2000; 175:363–370.27. Koutani A, Lechevallier E, André M, de Fromont M, Coulange C. [Contralateral perirenal metastasis of renal adenocarcinoma]. Prog Urol. 1997; 7:1002–1003.28. Nikolaos F, Panagiotis A, Konstantinos B, Vassilios S, Iraklis P. Distant ureteral metastasis from colon adenocarcinoma: report of a case and review of the literature. Case Rep Urol. 2014; 2014:196425.
Article29. Katsuno G, Kagawa S, Kokudo Y, Muraoka A, Tatemoto A, Sone Y, et al. Ureteral metastasis from appendiceal cancer: report of a case. Surg Today. 2005; 35:168–171.
Article30. Roy S, Baijal SS. Pancreatic adenocarcinoma presenting with ureteric metastases. Case report and review of literature. Clin Imaging. 1993; 17:99–103.
Article31. Haddad FS. Metastases to the ureter. Review of the world literature, and three new case reports. J Med Liban. 1999; 47:265–271.32. Stow B. IX. Fibrolymphosarcomata of both ureters metastatic to a primary lymphosarcomata of the anterior mediastinum of thymus origin. Ann Surg. 1909; 50:901–906.33. Maclean JT, Fowler VB. Pathology of tumors of the renal pelvis and ureter. J Urol. 1956; 75:384–415.
Article34. Presman D, Ehrlich L. Metastatic tumors of the ureter. J Urol. 1948; 59:312–325.
Article35. Cohen WM, Freed SZ, Hasson J. Metastatic cancer to the ureter: a review of the literature and case presentations. J Urol. 1974; 112:188–189.
Article36. Winalski CS, Lipman JC, Tumeh SS. Ureteral neoplasms. Radiographics. 1990; 10:271–283.
Article37. Fitch WP, Robinson JR, Radwin HW. Metastatic carcinoma of the ureter. Arch Surg. 1976; 111:874–876.
Article38. Marincek B, Scheidegger JR, Studer UE, Kraft R. Metastatic disease of the ureter: patterns of tumoral spread and radiologic findings. Abdom Imaging. 1993; 18:88–94.
Article39. Gelister JS, Falzon M, Crawford R, Chapple CR, Hendry WF. Urinary tract metastasis from renal carcinoma. Br J Urol. 1992; 69:250–252.
Article40. Morichetti D, Mazzucchelli R, Lopez-Beltran A, Cheng L, Scarpelli M, Kirkali Z, et al. Secondary neoplasms of the urinary system and male genital organs. BJU Int. 2009; 104:770–776.
Article41. Ramsey J, Beckman EN, Winters JC. Breast cancer metastatic to the urinary bladder. Ochsner J. 2008; 8:208–212.42. Kazama S, Kitayama J, Sunami E, Niimi A, Nomiya A, Homma Y, et al. Urethral metastasis from a sigmoid colon carcinoma: a quite rare case report and review of the literature. BMC Surg. 2014; 14:31.
Article43. Cheng CW, Wong WS, Chan LW, Lai FM. A rare cause of acute urinary retention: urethral metastasis from renal cell carcinoma. Int Urol Nephrol. 2004; 36:145–147.
Article44. Bhagavatula SK, Shyn PB. Image-guided renal interventions. Radiol Clin North Am. 2017; 55:359–371.
Article45. Rybicki FJ, Shu KM, Cibas ES, Fielding JR, vanSonnenberg E, Silverman SG. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003; 180:1281–1287.
Article46. Butros SR, McCarthy CJ, Karaosmanoğlu AD, Shenoy-Bhangle AS, Arellano RS. Feasibility and effectiveness of image-guided percutaneous biopsy of the urinary bladder. Abdom Imaging. 2015; 40:1838–1842.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characteristics of Multiple Primary Malignant Neoplasms Associated with the Urinary Tract Malignancy
- Radionuclide Imaging on Urinary Tract Obstruction
- The A.B.O. Blood Groups in Tumors of the Genitourinary Tract Among Korean
- Diagnostic Conundrum: Fever and Pyuria Preceding Diagnosis of Kawasaki Disease in Children
- Clinical Observation on Tumors of the Genito-urinary Tract