Korean J Radiol.
2018 Aug;19(4):682-691. 10.3348/kjr.2018.19.4.682.
Early Prediction of Response to Neoadjuvant Chemotherapy Using Dynamic Contrast-Enhanced MRI and Ultrasound in Breast Cancer
- Affiliations
-
- 1Department of Radiology, National Cancer Center, Goyang 10408, Korea.
- 2Department of Radiology, College of Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul 06591, Korea.
- 3Department of Surgery, College of Medicine, Bucheon St. Mary's Hospital, The Catholic University of Korea, Bucheon 14647, Korea. bjsong@catholic.ac.kr
- 4Department of Pathology, College of Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2413697
- DOI: http://doi.org/10.3348/kjr.2018.19.4.682
Abstract
OBJECTIVE
To determine the diagnostic performance of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and DCE ultrasound (DCE-US) for predicting response to neoadjuvant chemotherapy (NAC) in breast cancer patients.
MATERIALS AND METHODS
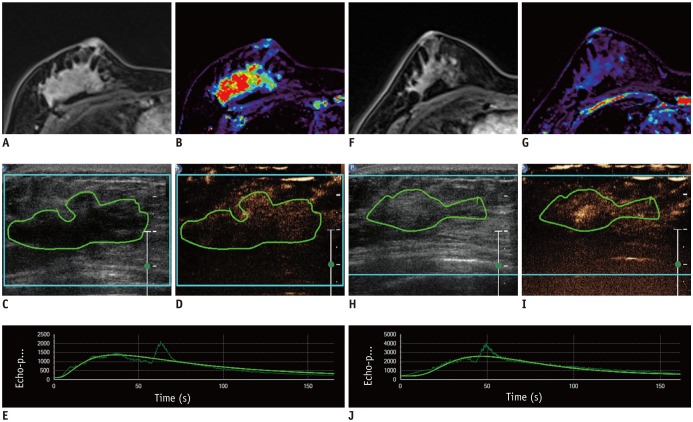
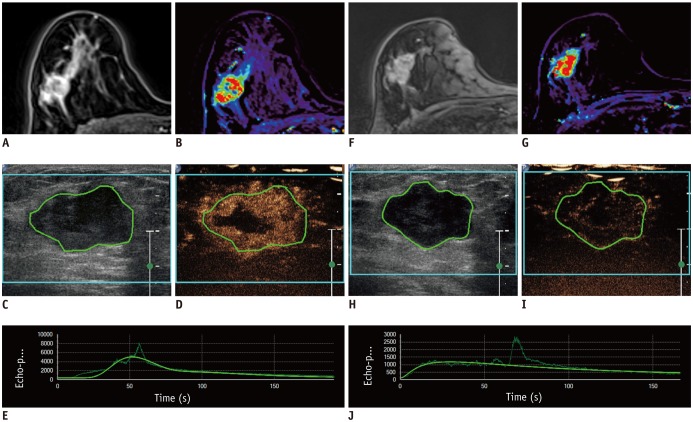
This Institutional Review Board-approved prospective study was performed between 2014 and 2016. Thirty-nine women with breast cancer underwent DCE-US and DCE-MRI before the NAC, follow-up DCE-US after the first cycle of NAC, and follow-up DCE-MRI after the second cycle of NAC. DCE-MRI parameters (transfer constant [Ktrans], reverse constant [kep], and leakage space [Ve]) were assessed with histograms. From DCE-US, peak-enhancement, the area under the curve, wash-in rate, wash-out rate, time to peak, and rise time (RT) were obtained. After surgery, all the imaging parameters and their changes were compared with histopathologic response using the Miller-Payne Grading (MPG) system. Data from minor and good responders were compared using Wilcoxon rank sum test, chi-square test, or Fisher's exact test. Receiver operating characteristic curve analysis was used for assessing diagnostic performance to predict good response.
RESULTS
Twelve patients (30.8%) showed a good response (MPG 4 or 5) and 27 (69.2%) showed a minor response (MPG 1-3). The mean, 25th, 50th, and 75th percentiles of Ktrans and Kep of post-NAC DCE-MRI differed between the two groups. These parameters showed fair to good diagnostic performance for the prediction of response to NAC (AUC 0.76-0.81, p ≤ 0.007). Among DCE-US parameters, the percentage change in RT showed fair prediction (AUC 0.71, p = 0.023).
CONCLUSION
Quantitative analysis of DCE-MRI and DCE-US was helpful for early prediction of response to NAC.
MeSH Terms
Figure
Cited by 1 articles
-
Kinetic Features of Invasive Breast Cancers on Computer-Aided Diagnosis Using 3T MRI Data: Correlation with Clinical and Pathologic Prognostic Factors
Sung Eun Song, Kyu Ran Cho, Bo Kyoung Seo, Ok Hee Woo, Seung Pil Jung, Deuk Jae Sung
Korean J Radiol. 2019;20(3):411-421. doi: 10.3348/kjr.2018.0587.
Reference
-
1. Marinovich ML, Sardanelli F, Ciatto S, Mamounas E, Brennan M, Macaskill P, et al. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast. 2012; 21:669–677. PMID: 22863284.
Article2. Lyou CY, Cho N, Kim SM, Jang M, Park JS, Baek SY, et al. Computer-aided evaluation of breast MRI for the residual tumor extent and response monitoring in breast cancer patients receiving neoadjuvant chemotherapy. Korean J Radiol. 2011; 12:34–43. PMID: 21228938.
Article3. Pickles MD, Gibbs P, Lowry M, Turnbull LW. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging. 2006; 24:843–847. PMID: 16916701.
Article4. Li SP, Padhani AR. Tumor response assessments with diffusion and perfusion MRI. J Magn Reson Imaging. 2012; 35:745–763. PMID: 22434697.
Article5. Padhani AR, Dzik-Jurasz A. Perfusion MR imaging of extracranial tumor angiogenesis. Top Magn Reson Imaging. 2004; 15:41–57. PMID: 15057172.
Article6. Knopp MV, Weiss E, Sinn HP, Mattern J, Junkermann H, Radeleff J, et al. Pathophysiologic basis of contrast enhancement in breast tumors. J Magn Reson Imaging. 1999; 10:260–266. PMID: 10508285.
Article7. Kim SH, Lee HS, Kang BJ, Song BJ, Kim HB, Lee H, et al. Dynamic contrast-enhanced MRI perfusion parameters as imaging biomarkers of angiogenesis. PLoS One. 2016; 11:e0168632. PMID: 28036342.
Article8. Kerbel RS, Klement G, Pritchard KI, Kamen B. Continuous low-dose anti-angiogenic/metronomic chemotherapy: from the research laboratory into the oncology clinic. Ann Oncol. 2002; 13:12–15. PMID: 11863092.9. Khalifa F, Soliman A, El-Baz A, Abou El-Ghar M, El-Diasty T, Gimel'farb G, et al. Models and methods for analyzing DCE-MRI: a review. Med Phys. 2014; 41:124301. PMID: 25471985.
Article10. Padhani AR, Hayes C, Assersohn L, Powles T, Makris A, Suckling J, et al. Prediction of clinicopathologic response of breast cancer to primary chemotherapy at contrast-enhanced MR imaging: initial clinical results. Radiology. 2006; 239:361–374. PMID: 16543585.
Article11. Ah-See ML, Makris A, Taylor NJ, Harrison M, Richman PI, Burcombe RJ, et al. Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2008; 14:6580–6589. PMID: 18927299.
Article12. Li X, Arlinghaus LR, Ayers GD, Chakravarthy AB, Abramson RG, Abramson VG, et al. DCE-MRI analysis methods for predicting the response of breast cancer to neoadjuvant chemotherapy: pilot study findings. Magn Reson Med. 2014; 71:1592–1602. PMID: 23661583.
Article13. Tudorica A, Oh KY, Chui SY, Roy N, Troxell ML, Naik A, et al. Early prediction and evaluation of breast cancer response to neoadjuvant chemotherapy using quantitative DCE-MRI. Transl Oncol. 2016; 9:8–17. PMID: 26947876.
Article14. Miyamoto Y, Ito T, Takada E, Omoto K, Hirai T, Moriyasu F. Efficacy of sonazoid (perflubutane) for contrast-enhanced ultrasound in the differentiation of focal breast lesions: phase 3 multicenter clinical trial. AJR Am J Roentgenol. 2014; 202:W400–W407. PMID: 24660739.
Article15. Caproni N, Marchisio F, Pecchi A, Canossi B, Battista R, D'Alimonte P, et al. Contrast-enhanced ultrasound in the characterisation of breast masses: utility of quantitative analysis in comparison with MRI. Eur Radiol. 2010; 20:1384–1395. PMID: 20033178.
Article16. Wan CF, Du J, Fang H, Li FH, Zhu JS, Liu Q. Enhancement patterns and parameters of breast cancers at contrast-enhanced US: correlation with prognostic factors. Radiology. 2012; 262:450–459. PMID: 22282183.
Article17. Cao X, Xue J, Zhao B. Potential application value of contrast-enhanced ultrasound in neoadjuvant chemotherapy of breast cancer. Ultrasound Med Biol. 2012; 38:2065–2071. PMID: 23062366.
Article18. Lee SC, Grant E, Sheth P, Garcia AA, Desai B, Ji L, et al. Accuracy of contrast-enhanced ultrasound compared with magnetic resonance imaging in assessing the tumor response after neoadjuvant chemotherapy for breast cancer. J Ultrasound Med. 2017; 36:901–911. PMID: 28150325.
Article19. Saracco A, Szabó BK, Tánczos E, Bergh J, Hatschek T. Contrast-enhanced ultrasound (CEUS) in assessing early response among patients with invasive breast cancer undergoing neoadjuvant chemotherapy. Acta Radiol. 2017; 58:394–402. PMID: 27461224.
Article20. Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999; 10:223–232. PMID: 10508281.
Article21. Just N. Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer. 2014; 111:2205–2213. PMID: 25268373.
Article22. Heo SH, Shin SS, Kim JW, Lim HS, Jeong YY, Kang WD, et al. Pre-treatment diffusion-weighted MR imaging for predicting tumor recurrence in uterine cervical cancer treated with concurrent chemoradiation: value of histogram analysis of apparent diffusion coefficients. Korean J Radiol. 2013; 14:616–625. PMID: 23901319.
Article23. Tranquart F, Mercier L, Frinking P, Gaud E, Arditi M. Perfusion quantification in contrast-enhanced ultrasound (CEUS)--ready for research projects and routine clinical use. Ultraschall Med. 2012; 33(Suppl 1):S31–S38. PMID: 22723027.24. Zink F, Kratzer W, Schmidt S, Oeztuerk S, Mason RA, Porzner M, et al. Comparison of two high-end ultrasound systems for contrast-enhanced ultrasound quantification of mural microvascularity in crohn's disease. Ultraschall Med. 2016; 37:74–81. PMID: 26251995.
Article25. Park CK, Jung WH, Koo JS. Pathologic evaluation of breast cancer after neoadjuvant therapy. J Pathol Transl Med. 2016; 50:173–180. PMID: 27068026.
Article26. Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003; 12:320–327. PMID: 14659147.
Article27. Cho N, Im SA, Park IA, Lee KH, Li M, Han W, et al. Breast cancer: early prediction of response to neoadjuvant chemotherapy using parametric response maps for MR imaging. Radiology. 2014; 272:385–396. PMID: 24738612.
Article28. Prevos R, Smidt ML, Tjan-Heijnen VC, van Goethem M, Beets-Tan RG, Wildberger JE, et al. Pre-treatment differences and early response monitoring of neoadjuvant chemotherapy in breast cancer patients using magnetic resonance imaging: a systematic review. Eur Radiol. 2012; 22:2607–2616. PMID: 22983282.
Article29. Corcioni B, Santilli L, Quercia S, Zamagni C, Santini D, Taffurelli M, et al. Contrast-enhanced US and MRI for assessing the response of breast cancer to neoadjuvant chemotherapy. J Ultrasound. 2008; 11:143–150. PMID: 23396645.
Article30. Dietrich CF, Averkiou MA, Correas JM, Lassau N, Leen E, Piscaglia F. An EFSUMB introduction into dynamic contrast-enhanced ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012; 33:344–351. PMID: 22843433.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment Response Evaluation of Breast Cancer after Neoadjuvant Chemotherapy and Usefulness of the Imaging Parameters of MRI and PET/CT
- Response Evaluation to Neoadjuvant Chemotherapy in Advanced Breast Cancer: Comparison of MRI and Positron Emission Tomography/CT (RECIST 1.1 versus PERCIST 1.0)
- Assessment of Neoadjuvant Treatment Response Using Automated Breast Ultrasound in Breast Cancer
- US-guided Clip Implantation for Tumor Localization in Breast Cancer Patients Who Undergo Neoadjuvant Chemotherapy: Feasibility Study
- Comparison of the Accuracy of Mammography, Ultrasonography and MRI for Evaluating Residual Tumor after Neoadjuvant Chemotherapy for Breast Cancer