Int J Stem Cells.
2018 Jun;11(1):48-60. 10.15283/ijsc17062.
Transcriptional Profiling of Mesenchymal Stem Cells Identifies Distinct Neuroimmune Pathways Altered by CNS Disease
- Affiliations
-
- 1Department of Neurosciences, Case Western Reserve University School of Medicine, Cleveland, USA. rhm3@gwu.edu
- 2Department of Anatomy and Regenerative Biology, George Washington University School of Medicine and Health Sciences, Washington DC, USA.
- 3Department of Pharmacology, George Washington University School of Medicine and Health Sciences, Washington DC, USA.
- KMID: 2413500
- DOI: http://doi.org/10.15283/ijsc17062
Abstract
- BACKGROUND AND OBJECTIVES
Bone marrow mesenchymal stem cells (BM-MSCs) are an attractive cell based therapy in the treatment of CNS demyelinating diseases such as multiple sclerosis (MS). Preclinical studies demonstrate that BM-MSCs can effectively reduce clinical burden and enhance recovery in experimental autoimmune encephalomyelitis (EAE), a commonly used animal model of MS. However, a number of recent clinical trials have not shown significant functional benefit following BM-MSC infusion into MS patients. One possibility for the discrepancy between animal and human studies is the source of the cells, as recent studies suggest BM-MSCs from MS patients or animals with EAE lack reparative efficacy compared to naïve cells. We sought to define important transcriptional and functional differences between diseased and naïve MSCs.
METHODS AND RESULTS
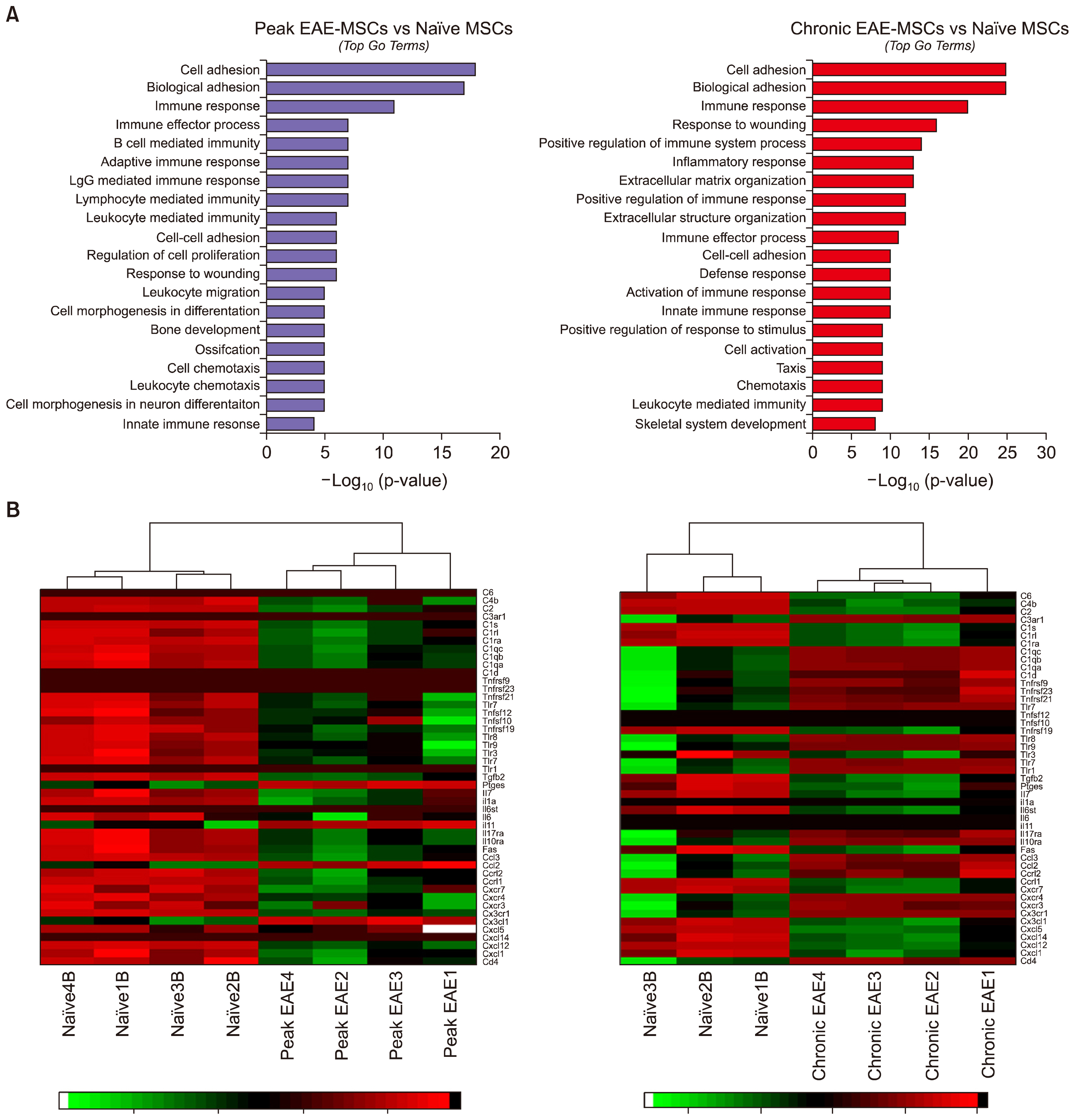
We utilized RNA Sequencing (RNA-Seq) to assess changes in gene expression between BM-MSCs derived from EAE animals and those derived from healthy controls. We show that EAE alters the expression of a large number of genes in BM-MSCs and changes in gene expression are more pronounced in chronic versus acute disease. Bioinformatic analysis revealed extensive perturbations in BM-MSCs in pathways related to inflammation and the regulation of neural cell development. These changes suggest that signals from EAE derived BM-MSCs inhibit rather than enhance remyelination, and in-vitro studies showed that conditioned medium from EAE MSCs fails to support the development of mature oligodendrocytes, the myelinating cells of the CNS.
CONCLUSIONS
These data provide insight into the failure of autologous BM-MSCs to promote recovery in MS and support the concept of utilizing non-autologous MSCs in future clinical trials.
Keyword
MeSH Terms
-
Acute Disease
Animals
Astrocytes
Bone Marrow
Central Nervous System Diseases*
Computational Biology
Culture Media, Conditioned
Demyelinating Diseases
Encephalomyelitis, Autoimmune, Experimental
Gene Expression
Humans
Inflammation
Mesenchymal Stromal Cells*
Models, Animal
Multiple Sclerosis
Myelin Sheath
Oligodendroglia
Sequence Analysis, RNA
Culture Media, Conditioned
Figure
Cited by 2 articles
-
Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Ace Card to Address Autoimmune Diseases
Hussein Baharlooi, Maryam Azimi, Zahra Salehi, Maryam Izad
Int J Stem Cells. 2019;13(1):13-23. doi: 10.15283/ijsc19108.Effect of Substrate Topography and Chemistry on Human Mesenchymal Stem Cell Markers: A Transcriptome Study
Bo Zhang, Naresh Kasoju, Qiongfang Li, Jinmin Ma, Aidong Yang, Zhanfeng Cui, Hui Wang, Hua Ye
Int J Stem Cells. 2019;12(1):84-94. doi: 10.15283/ijsc18102.
Reference
-
References
1. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008; 8:726–736. DOI: 10.1038/nri2395.
Article2. Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011; 9:12. DOI: 10.1186/1478-811X-9-12. PMID: 21569606. PMCID: 3117820.
Article3. Kassis I, Vaknin-Dembinsky A, Karussis D. Bone marrow mesenchymal stem cells: agents of immunomodulation and neuroprotection. Curr Stem Cell Res Ther. 2011; 6:63–68. DOI: 10.2174/157488811794480762.
Article4. Sargent A, Miller RH. MSC Therapeutics in chronic inflammation. Curr Stem Cell Rep. 2016; 2:168–173. DOI: 10.1007/s40778-016-0044-6.
Article5. Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med. 2013; 13:856–867. DOI: 10.2174/1566524011313050016. PMID: 23642066.
Article6. De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012; 12:574–591. DOI: 10.2174/156652412800619950. PMID: 22515979.
Article7. Rivera FJ, Couillard-Despres S, Pedre X, Ploetz S, Caioni M, Lois C, Bogdahn U, Aigner L. Mesenchymal stem cells instruct oligodendrogenic fate decision on adult neural stem cells. Stem Cells. 2006; 24:2209–2219. DOI: 10.1634/stemcells.2005-0614. PMID: 16763198.
Article8. Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, Miller RH. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009; 57:1192–1203. DOI: 10.1002/glia.20841. PMID: 19191336. PMCID: 2706928.
Article9. Jadasz JJ, Kremer D, Göttle P, Tzekova N, Domke J, Rivera FJ, Adjaye J, Hartung HP, Aigner L, Küry P. Mesenchymal stem cell conditioning promotes rat oligodendroglial cell maturation. PLoS One. 2013; 8:e71814. DOI: 10.1371/journal.pone.0071814. PMID: 23951248. PMCID: 3741203.
Article10. Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, Zaremba A, Miller RH. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat Neurosci. 2012; 15:862–870. DOI: 10.1038/nn.3109. PMID: 22610068. PMCID: 3427471.
Article11. Miller RH, Bai L, Lennon DP, Caplan AI. The potential of mesenchymal stem cells for neural repair. Discov Med. 2010; 9:236–242. PMID: 20350491.12. Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006; 129:1953–1971. DOI: 10.1093/brain/awl075.
Article13. Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005; 106:1755–1761. DOI: 10.1182/blood-2005-04-1496. PMID: 15905186.
Article14. Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005; 195:16–26. DOI: 10.1016/j.expneurol.2005.03.018. PMID: 15904921.
Article15. Zhang J, Li Y, Lu M, Cui Y, Chen J, Noffsinger L, Elias SB, Chopp M. Bone marrow stromal cells reduce axonal loss in experimental autoimmune encephalomyelitis mice. J Neurosci Res. 2006; 84:587–595. DOI: 10.1002/jnr.20962. PMID: 16773650.
Article16. Al Jumah MA, Abumaree MH. The immunomodulatory and neuroprotective effects of mesenchymal stem cells (MSCs) in experimental autoimmune encephalomyelitis (EAE): a model of multiple sclerosis (MS). Int J Mol Sci. 2012; 13:9298–9331. DOI: 10.3390/ijms13079298. PMID: 22942767. PMCID: 3430298.
Article17. Meamar R, Nematollahi S, Dehghani L, Mirmosayyeb O, Shayegannejad V, Basiri K, Tanhaei AP. The role of stem cell therapy in multiple sclerosis: An overview of the current status of the clinical studies. Adv Biomed Res. 2016; 5:46. DOI: 10.4103/2277-9175.178791. PMID: 27110543. PMCID: 4817403.
Article18. Sargent A, Bai L, Shano G, Karl M, Garrison E, Ranasinghe L, Planchon SM, Cohen J, Miller RH. CNS disease diminishes the therapeutic functionality of bone marrow mesenchymal stem cells. Exp Neurol. 2017; 295:222–232. DOI: 10.1016/j.expneurol.2017.06.013. PMID: 28602834. PMCID: 5536847.
Article19. Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008; 105:18460–18465. DOI: 10.1073/pnas.0809850105. PMID: 19015529. PMCID: 2587589.
Article20. Li Y, Lin F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood. 2012; 120:3436–3443. DOI: 10.1182/blood-2012-03-420612. PMID: 22966167. PMCID: 3482856.
Article21. Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010; 5:e10088. DOI: 10.1371/journal.pone.0010088. PMID: 20436665. PMCID: 2859930.
Article22. Wen S, Li H, Liu J. Dynamic signaling for neural stem cell fate determination. Cell Adh Migr. 2009; 3:107–117. DOI: 10.4161/cam.3.1.7602. PMID: 19262166. PMCID: 2675157.
Article23. Grinspan JB, Edell E, Carpio DF, Beesley JS, Lavy L, Pleasure D, Golden JA. Stage-specific effects of bone morphogenetic proteins on the oligodendrocyte lineage. J Neurobiol. 2000; 43:1–17. DOI: 10.1002/(SICI)1097-4695(200004)43:1<1::AID-NEU1>3.0.CO;2-0. PMID: 10756062.
Article24. Stipursky J, Gomes FC. TGF-beta1/SMAD signaling induces astrocyte fate commitment in vitro: implications for radial glia development. Glia. 2007; 55:1023–1033. DOI: 10.1002/glia.20522. PMID: 17549683.
Article25. Palazuelos J, Klingener M, Aguirre A. TGFβ signaling regulates the timing of CNS myelination by modulating oligodendrocyte progenitor cell cycle exit through SMAD3/4/FoxO1/Sp1. J Neurosci. 2014; 34:7917–7930. DOI: 10.1523/JNEUROSCI.0363-14.2014. PMID: 24899714. PMCID: 4044250.
Article26. Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol. 2004; 164:111–122. DOI: 10.1083/jcb.200308101. PMID: 14709544. PMCID: 2171962.
Article27. Auletta JJ, Bartholomew AM, Maziarz RT, Deans RJ, Miller RH, Lazarus HM, Cohen JA. The potential of mesenchymal stromal cells as a novel cellular therapy for multiple sclerosis. Immunotherapy. 2012; 4:529–547. DOI: 10.2217/imt.12.41. PMID: 22642335. PMCID: 3381871.
Article28. Bonab MM, Sahraian MA, Aghsaie A, Karvigh SA, Hosseinian SM, Nikbin B, Lotfi J, Khorramnia S, Motamed MR, Togha M, Harirchian MH, Moghadam NB, Alikhani K, Yadegari S, Jafarian S, Gheini MR. Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: an open label study. Curr Stem Cell Res Ther. 2012; 7:407–414. DOI: 10.2174/157488812804484648. PMID: 23061813.
Article29. Yamout B, Hourani R, Salti H, Barada W, El-Hajj T, Al-Kutoubi A, Herlopian A, Baz EK, Mahfouz R, Khalil-Hamdan R, Kreidieh NM, El-Sabban M, Bazarbachi A. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol. 2010; 227:185–189. DOI: 10.1016/j.jneuroim.2010.07.013. PMID: 20728948.
Article30. Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, Du MQ, Luan SL, Altmann DR, Thompson AJ, Compston A, Scott MA, Miller DH, Chandran S. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012; 11:150–156. DOI: 10.1016/S1474-4422(11)70305-2. PMID: 22236384. PMCID: 3279697.
Article31. Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010; 67:1187–1194. DOI: 10.1001/archneurol.2010.248. PMID: 20937945. PMCID: 3036569.
Article32. Dulamea A. Mesenchymal stem cells in multiple sclerosis - translation to clinical trials. J Med Life. 2015; 8:24–27. PMID: 25914733. PMCID: 4397514.33. Mallam E, Kemp K, Wilkins A, Rice C, Scolding N. Characterization of in vitro expanded bone marrow-derived mesenchymal stem cells from patients with multiple sclerosis. Mult Scler. 2010; 16:909–918. DOI: 10.1177/1352458510371959. PMID: 20542920.
Article34. Mazzanti B, Aldinucci A, Biagioli T, Barilaro A, Urbani S, Dal Pozzo S, Amato MP, Siracusa G, Crescioli C, Manuelli C, Bosi A, Saccardi R, Massacesi L, Ballerini C. Differences in mesenchymal stem cell cytokine profiles between MS patients and healthy donors: implication for assessment of disease activity and treatment. J Neuroimmunol. 2008; 199:142–150. DOI: 10.1016/j.jneuroim.2008.05.006. PMID: 18562015.
Article35. Redondo J, Sarkar P, Kemp K, Virgo PF, Pawade J, Norton A, Emery DC, Guttridge MG, Marks DI, Wilkins A, Scolding NJ, Rice CM. Reduced cellularity of bone marrow in multiple sclerosis with decreased MSC expansion potential and premature ageing in vitro. Mult Scler. 2017; 1352458517711276. DOI: 10.1177/1352458517711276. PMID: 28548004.
Article36. de Oliveira GL, de Lima KW, Colombini AM, Pinheiro DG, Panepucci RA, Palma PV, Brum DG, Covas DT, Simões BP, de Oliveira MC, Donadi EA, Malmegrim KC. Bone marrow mesenchymal stromal cells isolated from multiple sclerosis patients have distinct gene expression profile and decreased suppressive function compared with healthy counterparts. Cell Transplant. 2015; 24:151–165. DOI: 10.3727/096368913X675142.
Article37. Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff H, Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013; 11:146. DOI: 10.1186/1741-7015-11-146. PMID: 23758701. PMCID: 3694028.
Article38. Cohen JA. Mesenchymal stem cell transplantation in multiple sclerosis. J Neurol Sci. 2013; 333:43–49. DOI: 10.1016/j.jns.2012.12.009. PMID: 23294498. PMCID: 3624046.
Article39. Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002; 67:451–467. DOI: 10.1016/S0301-0082(02)00058-8. PMID: 12385864.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Mesenchymal Stem Cells in Patients with Nontraumatic Osteonecrosis of the Femoral Head
- Insights into the signal transduction pathways of mouse lung type II cells revealed by transcription factor profiling in the transcriptome
- Recent Trends and Strategies in Stem Cell Therapy for Alzheimer's Disease
- Stem Cells and Niemann Pick Disease
- Nineteen Types of WNT Genes Mediate The Differentiation of C3H10T1/2 Cell Lines