Int J Stem Cells.
2018 Jun;11(1):13-25. 10.15283/ijsc18033.
Enhancement of Replication and Differentiation Potential of Human Bone Marrow Stem Cells by Nicotinamide Treatment
- Affiliations
-
- 1Department of Life Science, University of Seoul, Seoul, Korea. eeshwang@uos.ac.kr
- KMID: 2413497
- DOI: http://doi.org/10.15283/ijsc18033
Abstract
- BACKGROUND AND OBJECTIVES
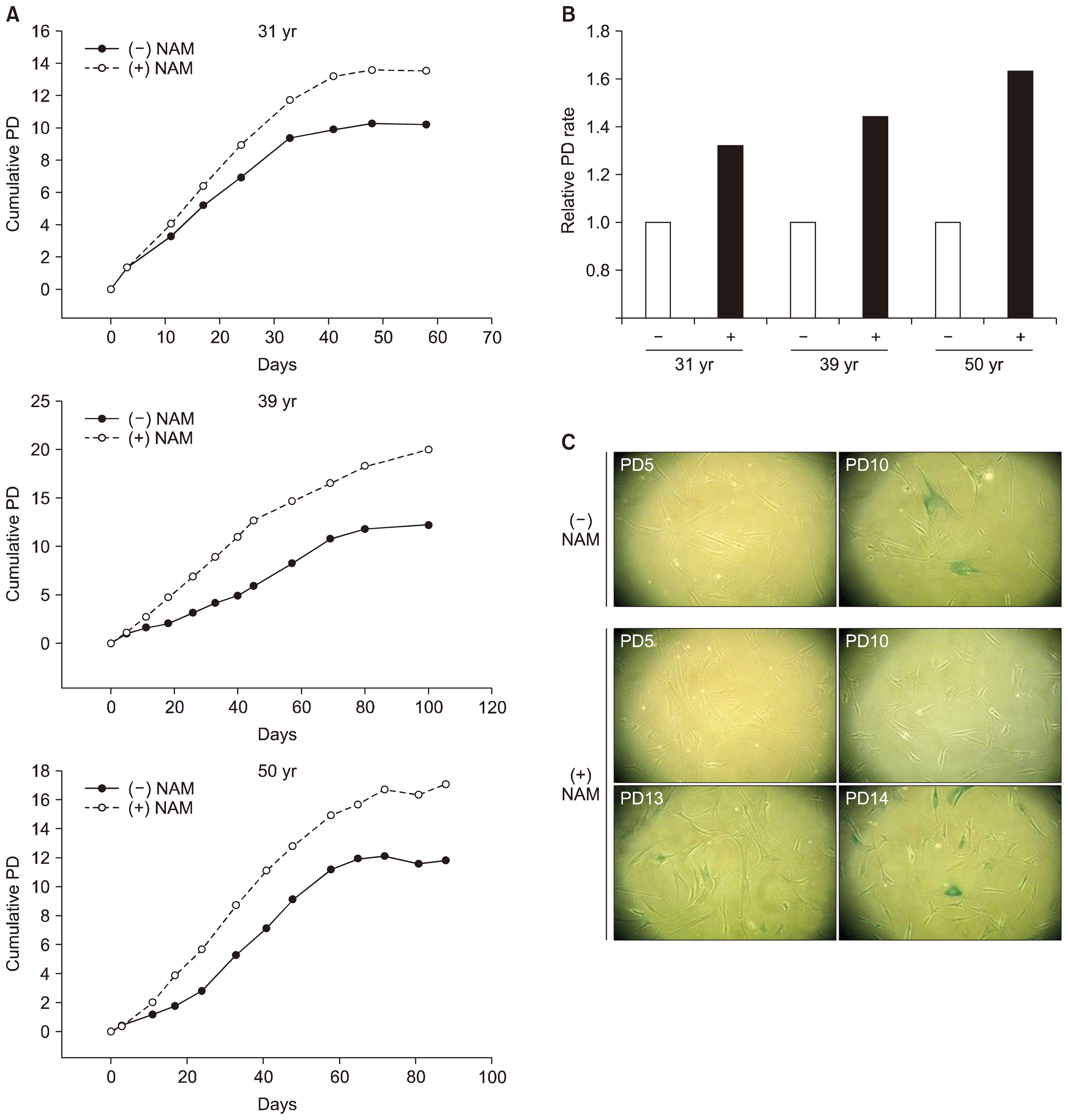
Therapies using mesenchymal stem cells (MSCs) generally require substantial expansion of cell populations. However, the replicative life span of MSCs is limited and their multipotency declines over continued passages, imposing a limitation on their application especially in aged individuals. In an effort to increase MSC life span, we tested the effects of nicotinamide (NAM), a precursor of NAD+ that has been shown to reduce reactive oxygen species generation and delay the onset of replicative senescence in fibroblasts.
METHODS
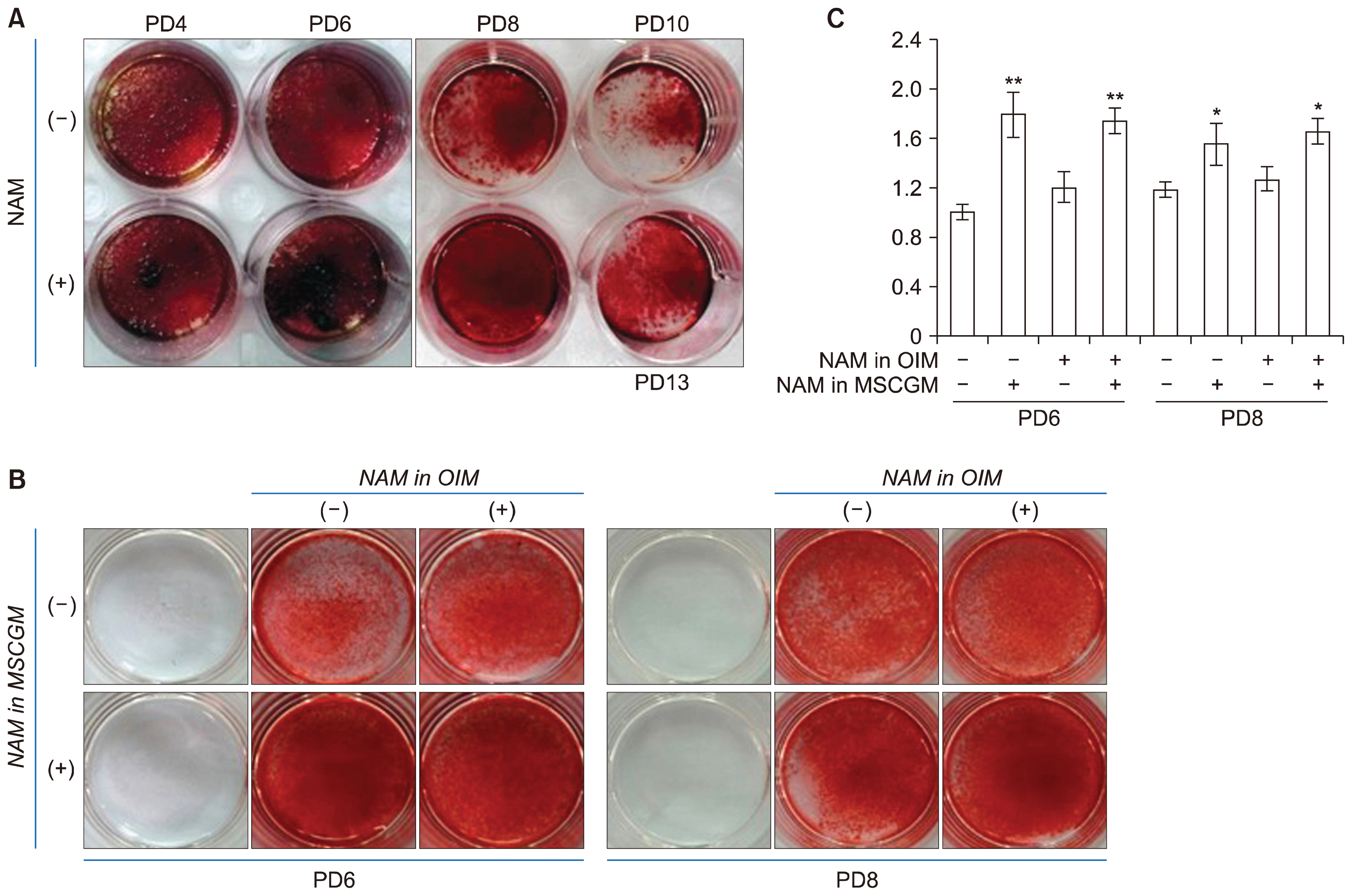
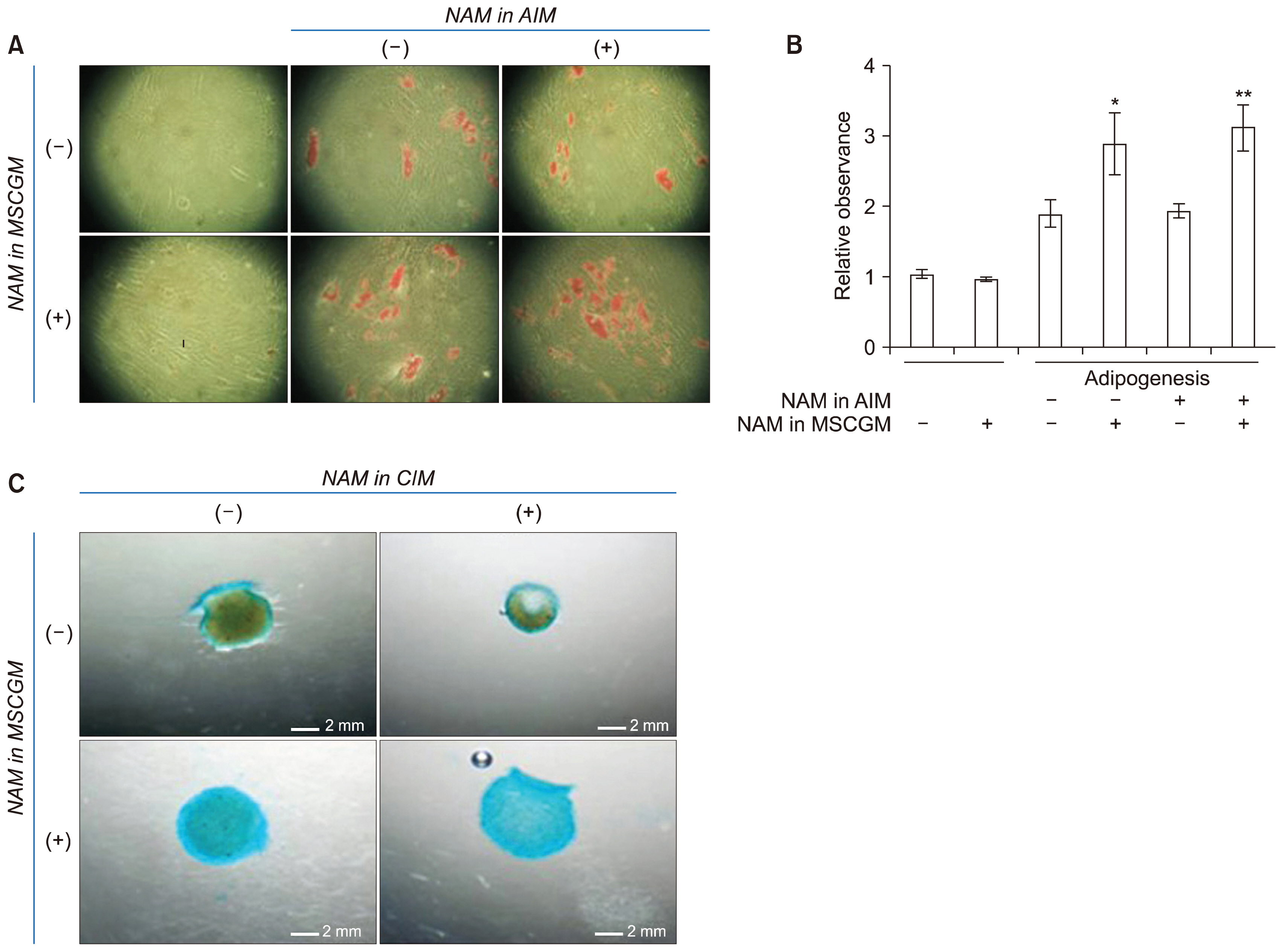
Bone marrow stem cells (BMSCs) from healthy donors were cultivated in the presence of 5 mM NAM until the end of their life span. The levels of proliferation and differentiation to osteogenic, adipogenic, and chondrogenic lineages of BMSCs were compared between populations incubated in the absence or presence of NAM.
RESULTS
The replicative life span was substantially increased with a significant delay in the onset of senescence, and differentiation to all tested lineages was increased. Furthermore, differentiation was sustained and the adipogenic switch from osteogenesis to adipogenesis was attenuated in late-passage BMSCs.
CONCLUSIONS
NAM could be considered as an important biological agent to expand and sustain the multipotency of BMSCs and thus broaden the application of stem cells in cell therapies.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Monitoring Glutathione Dynamics and Heterogeneity in Living Stem Cells
Eui Man Jeong, Ji-Woong Shin, Jisun Lim, Ju Hwan Kim, Hyewon Kang, Yingfu Yin, Hye-Mi Kim, YongHwan Kim, Sun-Gi Kim, Heun-Soo Kang, Dong-Myung Shin, Kihang Choi, In-Gyu Kim
Int J Stem Cells. 2019;12(2):367-379. doi: 10.15283/ijsc18151.
Reference
-
References
1. ClinicalTrials.gov [Internet]. Bethesda: U.S. National Library of Medicine;2000. Feb. 29. [(updated 2017 Dec 18) cited 2018 March 2]. Available from: https://clinicaltrials.gov/.2. Miura Y. Human bone marrow mesenchymal stromal/stem cells: current clinical applications and potential for hematology. Int J Hematol. 2016; 103:122–128. DOI: 10.1007/s12185-015-1920-z.
Article3. Hwang ES, Ok JS, Song S. Chemical and physical approaches to extend the replicative and differentiation potential of stem cells. Stem Cell Rev. 2016; 12:315–326. DOI: 10.1007/s12015-016-9652-x. PMID: 27085715.
Article4. Campisi J, Kim SH, Lim CS, Rubio M. Cellular senescence, cancer and aging: the telomere connection. Exp Gerontol. 2001; 36:1619–1637. DOI: 10.1016/S0531-5565(01)00160-7. PMID: 11672984.
Article5. Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive sub-cultivation and following cryopreservation. J Cell Biochem. 1997; 64:278–294. DOI: 10.1002/(SICI)1097-4644(199702)64:2<278::AID-JCB11>3.0.CO;2-F. PMID: 9027588.
Article6. Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hematol. 2000; 28:707–715. DOI: 10.1016/S0301-472X(00)00160-0. PMID: 10880757.
Article7. Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003; 17:160–170. DOI: 10.1038/sj.leu.2402763. PMID: 12529674.
Article8. Zaim M, Karaman S, Cetin G, Isik S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann Hematol. 2012; 91:1175–1186. DOI: 10.1007/s00277-012-1438-x. PMID: 22395436.
Article9. Brohlin M, Kingham PJ, Novikova LN, Novikov LN, Wiberg M. Aging effect on neurotrophic activity of human mesenchymal stem cells. PLoS One. 2012; 7:e45052. DOI: 10.1371/journal.pone.0045052. PMID: 23028757. PMCID: 3444498.
Article10. Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008; 129:163–173. DOI: 10.1016/j.mad.2007.12.002. PMID: 18241911.
Article11. von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002; 27:339–344. DOI: 10.1016/S0968-0004(02)02110-2. PMID: 12114022.
Article12. Lenaz G. Role of mitochondria in oxidative stress and ageing. Biochim Biophys Acta. 1998; 1366:53–67. DOI: 10.1016/S0005-2728(98)00120-0. PMID: 9714734.
Article13. Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells. 2008; 26:960–968. DOI: 10.1634/stemcells.2007-0509. PMID: 18218821.
Article14. Zhang Y, Marsboom G, Toth PT, Rehman J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS One. 2013; 8:e77077. DOI: 10.1371/journal.pone.0077077. PMID: 24204740. PMCID: 3800007.
Article15. Kim KS, Choi HW, Yoon HE, Kim IY. Reactive oxygen species generated by NADPH oxidase 2 and 4 are required for chondrogenic differentiation. J Biol Chem. 2010; 285:40294–40302. DOI: 10.1074/jbc.M110.126821. PMID: 20952384. PMCID: 3001009.
Article16. Denu RA, Hematti P. Effects of oxidative stress on mesenchymal stem cell biology. Oxid Med Cell Longev. 2016; 2016:2989076. DOI: 10.1155/2016/2989076. PMID: 27413419. PMCID: 4928004.
Article17. Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014; 24:464–471. DOI: 10.1016/j.tcb.2014.04.002. PMID: 24786309. PMCID: 4112140.18. Hwang ES, Song SB. Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator in cells. Cell Mol Life Sci. 2017; 74:3347–3362. DOI: 10.1007/s00018-017-2527-8. PMID: 28417163.
Article19. Kang HT, Lee HI, Hwang ES. Nicotinamide extends replicative lifespan of human cells. Aging Cell. 2006; 5:423–436. DOI: 10.1111/j.1474-9726.2006.00234.x. PMID: 16939485.
Article20. Jang SY, Kang HT, Hwang ES. Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J Biol Chem. 2012; 287:19304–19314. DOI: 10.1074/jbc.M112.363747. PMID: 22493485. PMCID: 3365962.21. Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, Kleijer WJ, DiMaio D, Hwang ES. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006; 5:187–195. DOI: 10.1111/j.1474-9726.2006.00199.x. PMID: 16626397.22. Geissler S, Textor M, Kühnisch J, Könnig D, Klein O, Ode A, Pfitzner T, Adjaye J, Kasper G, Duda GN. Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells. PLoS One. 2012; 7:e52700. DOI: 10.1371/journal.pone.0052700.
Article23. Song SB, Jang SY, Kang HT, Wei B, Jeoun UW, Yoon GS, Hwang ES. Modulation of mitochondrial membrane potential and ROS generation by nicotinamide in a manner independent of SIRT1 and mitophagy. Mol Cells. 2017; 40:503–514. PMID: 28736426. PMCID: 5547220.
Article24. Xu J, Li Z, Hou Y, Fang W. Potential mechanisms underlying the Runx2 induced osteogenesis of bone marrow mesenchymal stem cells. Am J Transl Res. 2015; 7:2527–2535.25. Köllmer M, Buhrman JS, Zhang Y, Gemeinhart RA. Markers are shared between adipogenic and osteogenic differentiated mesenchymal stem cells. J Dev Biol Tissue Eng. 2013; 5:18–25. DOI: 10.5897/JDBTE2013.0065. PMID: 24013643. PMCID: 3765027.
Article26. Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002; 16:2813–2828. DOI: 10.1101/gad.1017802. PMID: 12414734. PMCID: 187468.
Article27. Lee DH, Lim BS, Lee YK, Yang HC. Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol Toxicol. 2006; 22:39–46. DOI: 10.1007/s10565-006-0018-z. PMID: 16463018.
Article28. Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011; 14:537–544. DOI: 10.1016/j.cmet.2011.08.007. PMID: 21982713. PMCID: 3190168.
Article29. Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008; 3:e2213. DOI: 10.1371/journal.pone.0002213. PMID: 18493317. PMCID: 2374903.
Article30. Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE, Kadowitz PJ, Izadpanah R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012; 8:215–225. DOI: 10.1016/j.scr.2011.11.002. PMID: 22265741.
Article31. Yuan HF, Zhai C, Yan XL, Zhao DD, Wang JX, Zeng Q, Chen L, Nan X, He LJ, Li ST, Yue W, Pei XT. SIRT1 is required for long-term growth of human mesenchymal stem cells. J Mol Med (Berl). 2012; 90:389–400. DOI: 10.1007/s00109-011-0825-4.
Article32. Hwang ES, Hwang SY. Cellular NAD+ level: a key determinant of mitochondrial quality and health. Ann Geriatr Med Res. 2017; 21:149–157. DOI: 10.4235/agmr.2017.21.4.149.
Article33. Shakibaei M, Shayan P, Busch F, Aldinger C, Buhrmann C, Lueders C, Mobasheri A. Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: potential role of Runx2 deacetylation. PLoS One. 2012; 7:e35712. DOI: 10.1371/journal.pone.0035712. PMID: 22539994. PMCID: 3335081.
Article34. Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004; 429:771–776. DOI: 10.1038/nature02583. PMID: 15175761. PMCID: 2820247.
Article35. Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, Westphall MS, Pagliarini DJ, Prolla TA, Assadi-Porter F, Roy S, Denu JM, Coon JJ. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell. 2013; 49:186–199. DOI: 10.1016/j.molcel.2012.10.024. PMCID: 3704155.36. Dittenhafer-Reed KE, Richards AL, Fan J, Smallegan MJ, Fotuhi Siahpirani A, Kemmerer ZA, Prolla TA, Roy S, Coon JJ, Denu JM. SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab. 2015; 21:637–646. DOI: 10.1016/j.cmet.2015.03.007. PMID: 25863253. PMCID: 4393847.
Article37. Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY). 2010; 2:914–923. DOI: 10.18632/aging.100252.
Article38. Chen Y, Zhang J, Lin Y, Lei Q, Guan KL, Zhao S, Xiong Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011; 12:534–541. DOI: 10.1038/embor.2011.65.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Differential Potential of Stem Cells Following Their Origin: Subacromial Bursa, Bone Marrow, Umbilical Cord Blood
- Skeletal myogenic differentiation of human periodontal ligament stromal cells isolated from orthodontically extracted premolars
- Is Stem Cell-Based Therapy Going on or Out for Cardiac Disease?
- Exploring upregulated genes during osteogenic differentiation of hMSCs