J Vet Sci.
2016 Jun;17(2):217-224. 10.4142/jvs.2016.17.2.217.
Multi-voxel magnetic resonance spectroscopy of cerebral metabolites in healthy dogs at 1.5 Tesla
- Affiliations
-
- 1Ian Animal Diagnostic Center, Seoul 06014, Korea.
- 2College of Veterinary Medicine, Kyungpook National University, Daegu 41566, Korea.
- 3College of Veterinary Medicine and Research Institute of Veterinary Medicine, Chungnam National University, Daejeon 34134, Korea. hjchoi@cnu.ac.kr
- KMID: 2413171
- DOI: http://doi.org/10.4142/jvs.2016.17.2.217
Abstract
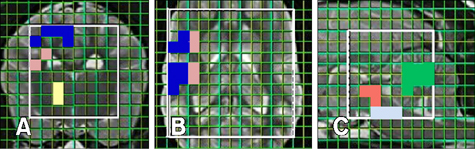
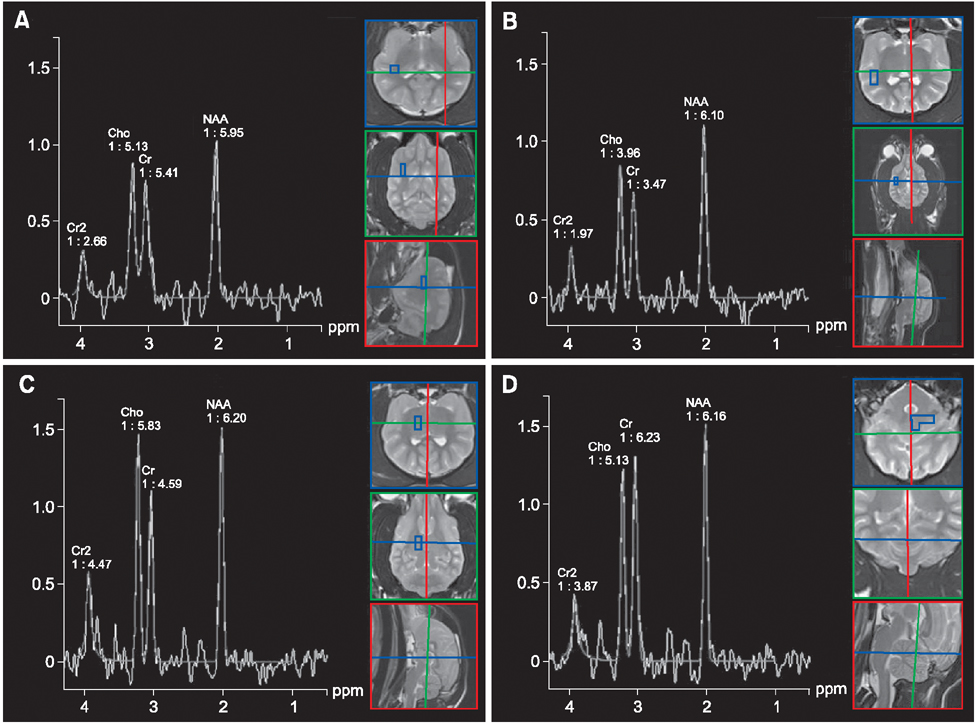
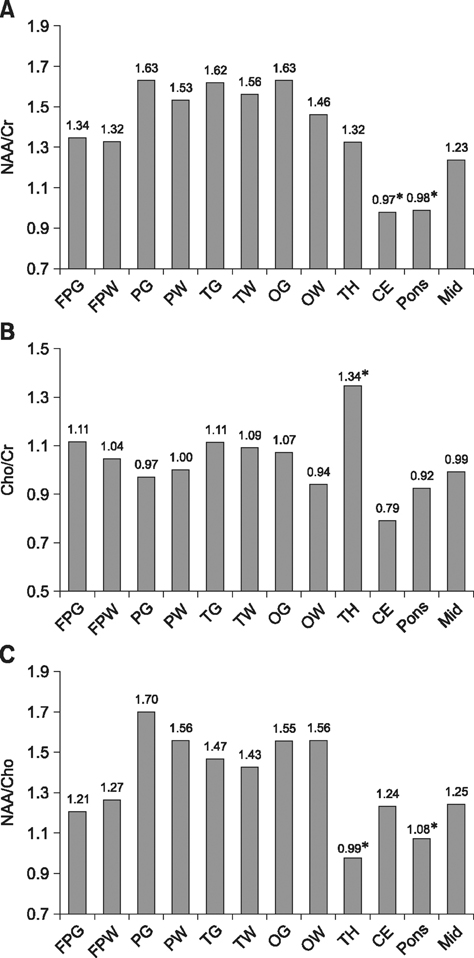
- This study was conducted to measure the difference in levels of cerebral metabolites in the right and left hemispheres, gray (GM) and white matter (WM), imaging planes, and anatomical regions of healthy dogs to establish normal variations. Eight male Beagle dogs (1 to 4 years of age; mean age, 2 years) with no evidence of neurologic disease were studied. Using the multi-voxel technique on a 1.5 Tesla magnetic resonance imaging scanner, metabolite values (N-acetyl aspartate [NAA], choline [Cho], creatine [Cr]) were obtained from the frontoparietal WM, parietal GM, temporal GM, occipital GM, thalamus, cerebellum, mid-brain, and pons. There was no significant difference in levels of these metabolites between the right and left in any locations or between the GM and WM in the cerebral hemispheres. However, there were significant differences in metabolite ratios within imaging planes. The NAA/Cr was lower in the cerebellum than other regions and the thalamus had a higher Cho/Cr and lower NAA/Cho ratio than in other regions. The spectral and metabolic values will provide a useful internal reference for clinical practice and research involving multi-voxel magnetic resonance spectroscopy. Measurement of metabolite values in the transverse plane is recommended for comparing levels of regional metabolites.
Keyword
MeSH Terms
Figure
Reference
-
1. Angelie E, Bonmartin A, Boudraa A, Gonnaud PM, Mallet JJ, Sappey-Marinier D. Regional differences and metabolic changes in normal aging of the human brain: proton MR spectroscopic imaging study. AJNR Am J Neuroradiol. 2001; 22:119–127.2. Baker EH, Basso G, Barker PB, Smith MA, Bonekamp D, Horská A. Regional apparent metabolite concentrations in young adult brain measured by 1H MR spectroscopy at 3 Tesla. J Magn Reson Imaging. 2008; 27:489–499.
Article3. Barker PB, Lin DDM. In vivo proton MR spectroscopy of the human brain. Prog Nucl Magn Reson Spectrosc. 2006; 49:99–128.
Article4. Barker PB, Szopinski K, Horská A. Metabolic heterogeneity at the level of the anterior and posterior commissures. Magn Reson Med. 2000; 43:348–354.
Article5. Barreiro CJ, Williams JA, Fitton TP, Lange MS, Blue ME, Kratz L, Barker PB, Degaonkar M, Gott VL, Troncoso JC, Johnston MV, Baumgartner WA. Noninvasive assessment of brain injury in a canine model of hypothermic circulatory arrest using magnetic resonance spectroscopy. Ann Thorac Surg. 2006; 81:1593–1598.
Article6. Bonavita S, Di Salle F, Tedeschi G. Proton MRS in neurological disorders. Eur J Radiol. 1999; 30:125–131.
Article7. Carrera I, Richter H, Meier D, Kircher PR, Dennler M. Regional metabolite concentrations in the brain of healthy dogs measured by use of short echo time, single voxel proton magnetic resonance spectroscopy at 3.0 Tesla. Am J Vet Res. 2015; 76:129–141.
Article8. Doelken MT, Mennecke A, Stadlbauer A, Kloska S, Struffert T, Engelhorn T, Thuerauf N, Doerfler A, Stefan H, Hammen T. Multi-voxel magnetic resonance spectroscopy of cerebral metabolites in healthy adults at 3 Tesla. Acad Radiol. 2009; 16:1493–1501.
Article9. Drost DJ, Riddle WR, Clarke GD. Proton magnetic resonance spectroscopy in the brain: report of AAPM MR Task Group #9. Med Phys. 2002; 29:2177–2197.
Article10. Gonen O, Gruber S, Li BS, Mlynárik V, Moser E. Multivoxel 3D proton spectroscopy in the brain at 1.5 versus 3.0 T: signal-to-noise ratio and resolution comparison. AJNR Am J Neuroradiol. 2001; 22:1727–1731.11. Hetherington H, Petroff O, Jackson GD, Kuzniecky RI, Briellmann RS, Wellard RM. Magnetic Resonance Spectroscopy. In : Kuzniecky RI, Jackson GD, editors. Magnetic Resonance in Epilepsy: Neuroimaging Techniques. 2nd ed. San Diego: Elsevier Academic Press;2005. p. 333–384.12. Jacobs MA, Horská A, van Zijl PC, Barker PB. Quantitative proton MR spectroscopic imaging of normal human cerebellum and brain stem. Magn Reson Med. 2001; 46:699–705.
Article13. Kang BT, Jang DP, Lee JH, Jung DI, Gu SH, Lim CY, Kim YB, Quan FS, Kim HJ, Woo EJ, Cho ZH, Park HM. Detection of cerebral metabolites in a canine model of ischemic stroke using 1H magnetic resonance spectroscopy. Res Vet Sci. 2009; 87:300–306.
Article14. Komoroski RA, Heimberg C, Cardwell D, Karson CN. Effects of gender and region on proton MRS of normal human brain. Magn Reson Imaging. 1999; 17:427–433.
Article15. Krukowski P, Podgórski P, Guziński M, Szewczyk P, Sasiadek M. Analysis of the brain proton magnetic resonance spectroscopy - differences between normal grey and white matter. Pol J Radiol. 2010; 75:22–26.16. Martin-Vaquero P, da Costa RC, Echandi RL, Sammet CL, Knopp MV, Sammet S. Magnetic resonance spectroscopy of the canine brain at 3.0 T and 7.0 T. Res Vet Sci. 2012; 93:427–429.17. Mascalchi M, Brugnoli R, Guerrini L, Belli G, Nistri M, Politi LS, Gavazzi C, Lolli F, Argenti G, Villari N. Singlevoxel long TE 1H-MR spectroscopy of the normal brainstem and cerebellum. J Magn Reson Imaging. 2002; 16:532–537.
Article18. Maudsley AA, Domenig C, Govind V, Darkazanli A, Studholme C, Arheart K, Bloomer C. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magn Reson Med. 2009; 61:548–559.
Article19. Nagae-Poetscher LM, Bonekamp D, Barker PB, Brant LJ, Kaufmann WE, Horská A. Asymmetry and gender effect in functionally lateralized cortical regions: a proton MRS imaging study. J Magn Reson Imaging. 2004; 19:27–33.
Article20. Noworolski SM, Nelson SJ, Henry RG, Day MR, Wald LL, Star-Lack J, Vigneron DB. High spatial resolution 1H-MRSI and segmented MRI of cortical gray matter and subcortical white matter in three regions of the human brain. Magn Reson Med. 1999; 41:21–29.
Article21. Ober CP, Warrington CD, Feeney DA, Jessen CR, Steward S. Optimizing a protocol for 1H-magnetic resonance spectroscopy of the canine brain at 3T. Vet Radiol Ultrasound. 2013; 54:149–158.
Article22. Ono K, Kitagawa M, Ito D, Tanaka N, Watari T. Regional variations and age-related changes detected with magnetic resonance spectroscopy in the brain of healthy dogs. Am J Vet Res. 2014; 75:179–186.
Article23. Ostojic J, Kozic D, Lucic M, Konstantinovic J, Covickovic-Sternic N, Pavlovic A, Bogdanovic-Stojanovic D, Semnic R. Multivoxel MRS: right frontal parafalcine cortex - area of neurobiochemical gender differentiation? Neuro Endocrinol Lett. 2011; 32:683–687.24. Pouwels PJ, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med. 1998; 39:53–60.
Article25. Rudkin TM, Arnold DL. Proton magnetic resonance spectroscopy for the diagnosis and management of cerebral disorders. Arch Neurol. 1999; 56:919–926.
Article26. Safriel Y, Pol-Rodriguez M, Novotny EJ, Rothman DL, Fulbright RK. Reference values for long echo time MR spectroscopy in healthy adults. AJNR Am J Neuroradiol. 2005; 26:1439–1445.27. Salibi N, Brown MA. MRS localization techniques. Clinical MR spectroscopy: First Principles. 1st ed. New York: John Wiley Sons;1998. p. 73–95.28. Schuff N, Ezekiel F, Gamst AC, Amend DL, Capizzano AA, Maudsley AA, Weiner MW. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn Reson Med. 2001; 45:899–907.
Article29. Soares DP, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin Radiol. 2009; 64:12–21.
Article30. Stadler KL, Ober CP, Feeney DA, Jessen CR. Multivoxel proton magnetic resonance spectroscopy of inflammatory and neoplastic lesions of the canine brain at 3.0 T. Am J Vet Res. 2014; 75:982–989.
Article31. van der Graaf M. In vivo magnetic resonance spectroscopy: basic methodology and clinical applications. Eur Biophys J. 2010; 39:527–540.
Article32. Wang Y, Li SJ. Differentiation of metabolic concentrations between gray matter and white matter of human brain by in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 1998; 39:28–33.
Article33. Warrington CD, Feeney DA, Ober CP, Jessen CR, Steward SM, Armién AG, Fletcher TF. Relative metabolite concentrations and ratios determined by use of 3-T regionspecific proton magnetic resonance spectroscopy of the brain of healthy Beagles. Am J Vet Res. 2013; 74:1291–1303.
Article34. Wiedermann D, Schuff N, Matson GB, Soher BJ, Du AT, Maudsley AA, Weiner MW. Short echo time multislice proton magnetic resonance spectroscopic imaging in human brain: metabolite distributions and reliability. Magn Reson Imaging. 2001; 19:1073–1080.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alteration Analysis of Normal Human Brain Metabolites with Variation of SENSE and NEX in 3T Multi Voxel Spectroscopy
- Usefulness of Proton MR Spectroscopy in Acute Cerebral Infarction: An Experimental and Clinical Study
- Investigation of Varied MR Spectra by TE and Metabolite Amount in the Localized Voxel using the MR Cone-shape Phantom
- The Change of Cerebral Metabolites in Patients with Parkinson's Disease Measured by Proton Magnetic Resonance Spectroscopy
- Cerebrospinal fluid flow in normal beagle dogs analyzed using magnetic resonance imaging